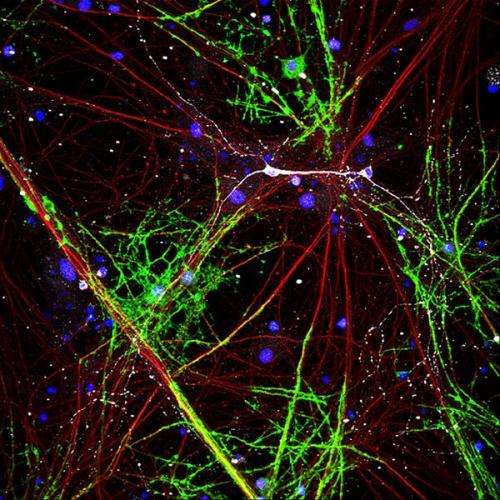
Oligodendrocytes myelinating glutamatergic but not interneurons. Credit: Plos Biology
(Medical Xpress)—A major question regarding how axons acquire a coat of myelin, is the role of spiking activity. It is known that in culture systems oligodendroctyes will at least try to wrap anything that feels like an axon—even dead axons and artificial tubes. As axons acquire additional layers of myelin they conduct signals faster, and presumably become more efficient. It would therefore seem logical that the nervous system should apportion the most myelin to those neurons that are seeing the greatest activity. In that way the brain gets the most bang for its buck, energetically speaking. A new study in PLOS Biology suggests that while myelination is in many cases activity-independent at first, neurons can significantly ramp things up by flipping various molecular switches, one which appears to be Neuregulin (NRG).
Recreating the process of myelination in culture can be difficult to get right. One recipe that works well is to use embryonic dorsal root ganglion neurons from rats, grow them for two weeks, and then plate post-natal oligodendrocyte precurser cells (OCPs) on top of them. With this kind of setup, cells can be patch-clamped to record and control their electrical activity. Using this protocol, and various pharmacological manipulations, the researchers were able to show that the activity-dependent mode of myelination was acting through NMDA receptors on the oligodendrocytes.
When the cultured neurons were spiking, their axons puffed glutamate through whatever release machinery was available to them at that stage of development. The researches measured a sixfold increase of NMDA receptor currents in the oligodendrocytes under these conditions. This was correlated with a significant increase in the expression of myelin basic proteins, which reflected increased myelination. These results were all neat and tidy, but a larger problem lurked behind the scenes. Numerous other studies had shown conflicting results with regards to spiking activity and NMDA receptor activation on myelination. In several studies, there was no effect at all.
Of course we know there is no real conflict here—rats don't seem to be seizing up mid-stride—we just don't have the whole picture yet. The resolution to this paradox that the researchers provide, is that there are two modes of myleination with neuregulin controlling the transition. Previous studies have shown that this NRG increases the expression of NMDA receptor subunits, at least in neurons. When the researchers looked into it, they found that the sensitization by NRG was the reason for the sixfold NMDA current increase in oligodendrocytes. If NRG is causing myelination to become NMDA receptor dependent, many of the experimental conflicts in the literature evaporate.
Much remains to be discovered as far as how myelination happens at all. Chemical switches are nice to know about, but without an understanding of how they translate to actual physical mechanisms, we are really no further along. One recent idea put forward is that certain kinds of phase transitions are exploited to drive myelination. If such phase transitions are happening, one question might be, in what direction does myelination tend to occur both within an axon segment, and down the entire extent of the axon?
Spikes in axons would clearly provide some directionality to myelination, and may even provide some of the forcing. The brain, especially in development, is a touchy-feely place with direct sensing often acting as a prelude to more intimate chemical exchanges. Receptor-mediated events surely happen all the time, but when introduced via cytonemes, cilia, or plain old pseudopods, they can be made more likely, and more likely to last.
More information: Neuregulin and BDNF Induce a Switch to NMDA Receptor-Dependent Myelination by Oligodendrocytes, PLOS Biology, www.plosbiology.org/article/in … journal.pbio.1001743
Abstract
Myelination is essential for rapid impulse conduction in the CNS, but what determines whether an individual axon becomes myelinated remains unknown. Here we show, using a myelinating coculture system, that there are two distinct modes of myelination, one that is independent of neuronal activity and glutamate release and another that depends on neuronal action potentials releasing glutamate to activate NMDA receptors on oligodendrocyte lineage cells. Neuregulin switches oligodendrocytes from the activity-independent to the activity-dependent mode of myelination by increasing NMDA receptor currents in oligodendrocyte lineage cells 6-fold. With neuregulin present myelination is accelerated and increased, and NMDA receptor block reduces myelination to far below its level without neuregulin. Thus, a neuregulin-controlled switch enhances the myelination of active axons. In vivo, we demonstrate that remyelination after white matter damage is NMDA receptor-dependent. These data resolve controversies over the signalling regulating myelination and suggest novel roles for neuregulin in schizophrenia and in remyelination after white matter damage.
Journal information: PLoS Biology
© 2014 Medical Xpress