Split decision: Stem cell signal linked with cancer growth
Researchers at the University of California, San Diego School of Medicine have identified a protein critical to hematopoietic stem cell function and blood formation. The finding has potential as a new target for treating leukemia because cancer stem cells rely upon the same protein to regulate and sustain their growth.
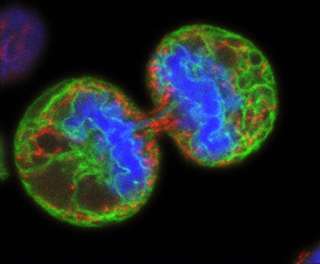
Hematopoietic stem cells give rise to all other blood cells. Writing in the February 2, 2014 advance online issue of Nature Genetics, principal investigator Tannishtha Reya, PhD, professor in the Department of Pharmacology, and colleagues found that a protein called Lis1 fundamentally regulates asymmetric division of hematopoietic stem cells, assuring that the stem cells correctly differentiate to provide an adequate, sustained supply of new blood cells.
Asymmetric division occurs when a stem cell divides into two daughter cells of unequal inheritance: One daughter differentiates into a permanently specialized cell type while the other remains undifferentiated and capable of further divisions.
"This process is very important for the proper generation of all the cells needed for the development and function of many normal tissues," said Reya. When cells divide, Lis1 controls orientation of the mitotic spindle, an apparatus of subcellular fibers that segregates chromosomes during cell division.
"During division, the spindle is attached to a particular point on the cell membrane, which also determines the axis along which the cell will divide," Reya said. "Because proteins are not evenly distributed throughout the cell, the axis of division, in turn, determines the types and amounts of proteins that get distributed to each daughter cell. By analogy, imagine the difference between cutting the Earth along the equator versus halving it longitudinally. In each case, the countries that wind up in the two halves are different."
When researchers deleted Lis1 from mouse hematopoietic stem cells, differentiation was radically altered. Asymmetric division increased and accelerated differentiation, resulting in an oversupply of specialized cells and an ever-diminishing reserve of undifferentiated stem cells, which eventually resulted in a bloodless mouse.
"What we found was that a large part of the defect in blood formation was due to a failure of stem cells to expand," said Reya. "Instead of undergoing symmetric divisions to generate two stem cell daughters, they predominantly underwent asymmetric division to generate more specialized cells. As a result, the mice were unable to generate enough stem cells to sustain blood cell production."
The scientists next looked at how cancer stem cells in mice behaved when the Lis1 signaling pathway was blocked, discovering that they too lost the ability to renew and propagate. "In this sense, the effect Lis1 has on leukemic self-renewal parallels its role in normal stem cell self-renewal," Reya said.
Reya said the findings shed new light on the fundamental regulators of cell growth both in normal development and in cancer.
"Our work shows that elimination of Lis1 potently inhibits cancer growth, and identifies Lis1 and other regulators of protein inheritance as a new class of molecules that could be targeted in cancer therapy."
In the long term, Reya noted, it remains to be determined whether inhibiting Lis1 in cancer cells would produce unacceptable consequences in normal cells as well. "A number of commonly used hemotherapy agents target the machinery that controls cell division. Although these agents can be toxic, their effects on cancer cells are much more potent than their effects on normal cells, and so they continue to be used. Agents that target Lis1 might be more specific and less toxic, which would give them significant clinical value."
More information: Lis1 regulates asymmetric division in hematopoietic stem cells and in leukemia, DOI: 10.1038/ng.2889