Roswell Park Cancer Institute (RPCI) has received U.S. Food and Drug Administration (FDA) approval to enroll patients in a phase II clinical trial that will use a unique and highly precise method of delivering interstitial photodynamic therapy (I-PDT) to recurrent tumors of the head and neck.
Most patients with recurrent and nonresectable head-and-neck cancer experience poor quality of life and a projected survival that is measured in months. I-PDT is an additional treatment option that may improve outcomes for patients who respond poorly to standard therapies.
"These patients have no effective treatment options," says Gal Shafirstein, DSc, principal investigator of the study and a member of RPCI's Departments of Cell Stress Biology and Head & Neck/Plastic & Reconstructive Surgery. "We hope to learn through this study whether we can improve their overall survival by adding interstitial photodynamic therapy to standard treatment approaches."
PDT, a treatment approach developed at RPCI, uses laser light to activate a nontoxic drug called a photosensitizer. The process works in three ways: it destroys cancer, shuts down blood vessels that "feed" the tumor, and prompts the immune system to kill cancer cells throughout the body. It is associated with mild side effects and can be combined with standard chemotherapy and surgery, and followed with radiation therapy.
Initiated by Dr. Shafirstein, the phase II clinical trial will use the photosensitizer Photofrin, manufactured by Pinnacle Biologics, Inc., which is funding the trial. The study will be conducted in collaboration with Thomas Foster, PhD, and Timothy Baran, PhD, both of the Department of Imaging Sciences at the University of Rochester Medical Center.
Treating physician Hassan Arshad, MD, a surgical oncologist in RPCI's Department of Head & Neck/Plastic & Reconstructive Surgery, will be responsible for all clinical aspects of the study.
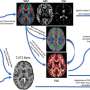
The clinical trial will borrow treatment-delivery techniques already used in the field of radiation medicine. Advanced treatment planning developed specifically for I-PDT will help the team to calculate the precise dose of laser light needed for each section of a tumor. That information will be used to treat tumors while minimizing damage to healthy tissue.
Patients enrolled in the study will undergo pretreatment imaging with computed tomography (CT). A three-dimensional image of the tumor will then be created with CT scans to determine the number and correct placement of the optical fibers that will deliver the laser light. The patients will receive Photofrin, followed by a two-day waiting period to allow the photosensitizer to be absorbed by the cancer cells. The treatment will be conducted while the patient is under general anesthesia. The optical fibers will be inserted through the skin and into the tumor at specific, predetermined points. A light-dosimetry system developed by Dr. Shafirstein in his RPCI lab will measure, in real time, the light being delivered by each optic fiber, giving the medical team the information needed to make necessary adjustments to meet the goals of the treatment plan.
"Collaborating with Roswell Park is an important step in expanding our clinical development platform for PDT with Photofrin," says Mark Thompson, chief executive officer of Concordia Healthcare Corp., parent company of Pinnacle Biologics. "Increased education, awareness and use of the dosimetry device, and the statistically significant outcomes we have achieved in other rare and hard-to-treat cancers, will inform this process and will, we hope, lead to better and more options for critically ill patients."
"We hope that these state-of-the-art approaches will maximize the potential benefit of this therapy for patients with recurrent head and neck tumors," Dr. Shafirstein adds.
Provided by Roswell Park Cancer Institute