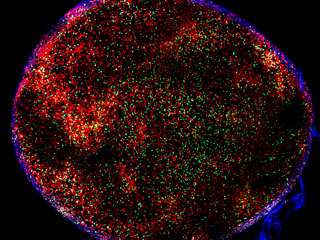
As the immune system fights an infection, it selects B cells that target the pathogen. A new study helps explain how the highest affinity B cells are selected. Above, red B cells in a lymph node form germinal centers where selection takes place. T cells, critical in this process, are green.
(Medical Xpress)—Over the weeks following an invasion by a disease-causing microbe, the human immune system fine tunes its defenses, producing proteins called antibodies that are ever more precisely targeted at the invader. New research in Michel Nussenzweig's Laboratory of Molecular Immunology helps explain how the immune system accomplishes this, suggesting new ways by which the body could be trained to fight disease.
"We have understood this process, called affinity maturation, in broad strokes for decades; however, the mechanistic details have remained more elusive," says Alex Gitlin, a graduate student in the lab and the first author of a study that delves into these mechanisms. "Our research helps explain how the immune system selects B cells that produce antibodies with a high affinity for a pathogen."
B cells are specialized immune cells that make antibodies that bind to foreign molecules, called antigens, on the surface of microbes. Prior to an infection, the body has a diverse repertoire of B cells, each capable of producing a unique antibody. During an infection or vaccination, B cells whose antibodies bind the antigen form microanatomical structures called germinal centers in the lymph nodes and spleen.
Within germinal centers, the B cells evolve. The gene responsible for producing their antibodies mutates rapidly, one million-fold faster than the normal rate of mutation in the human body, and the cells proliferate. Then, the B cells capable of producing antibodies with the highest affinity to the antigen are selected.
"These cycles of mutation, proliferation and selection happen over and over again. Over time, a few lines of high affinity B cells come to dominate the germinal centers," Gitlin says. Their current findings shed light on how the higher affinity B cells out-compete lower affinity cells.
Ultimately, the high affinity B cells survive and can protect the body should the same microbe be re-encountered later on. Vaccines take advantage of this process, using weakened versions of pathogens to imitate an infection. As a result, a better understanding of how the immune system adapts its defenses will help with vaccine development, including one against HIV.
In research described this week in Nature, Gitlin and colleagues at Rockefeller looked at what happens to B cells during their time in the two zones of the germinal center. In the "dark" zone, B cells mutate and proliferate before traveling to the "light" zone, where those with antibodies that bind best to the microbial antigens are selected. The B cells then continue to divide, returning to the dark zone.
While in the light zone, the B cells pick up bits of antigen from the invader and display them on their surface. The key finding is that specialized T cells in the light zone recognize higher affinity B cells based on the amount of antigen those B cells display. In response, the T cells provide a stronger signal to those higher affinity B cells, instructing them to stay longer in the dark zone and undergo a greater number of cell divisions.
The research team used a combination of techniques that allowed them to measure the history of cell division in the germinal center, as well as where each cell division is initiated, and how long the B cells spent in the dark zone.
The repeated process of hypermutation and selection creates a feed-forward loop, says Gitlin. "When a B cell is selected as being higher affinity, it is instructed by the T cell to divide a greater number of times, and through more cell divisions, they accumulate more mutations."
The importance of antibody hypermutation is best exemplified by HIV. The HIV virus mutates rapidly, but through a large amount of hypermutation and selection, the immune system is capable of producing broad and potent antibodies against it. Previous work in the lab has identified antibodies from the blood of patients to broadly neutralize variants of HIV.
More information: "Clonal selection in the germinal centre by regulated proliferation and hypermutation." Alexander D. Gitlin, et al. Nature (2014) DOI: 10.1038/nature13300. Received 17 January 2014 Accepted 01 April 2014 Published online 04 May 2014
Journal information: Nature
Provided by Rockefeller University