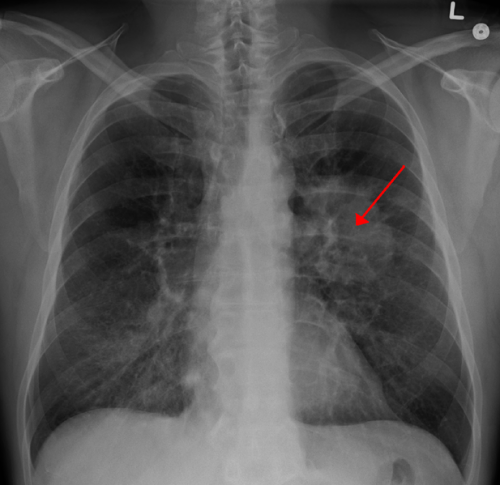
Lung CA seen on CXR. Credit: CC BY-SA 4.0 James Heilman, MD/Wikipedia
Moffitt Cancer Center researchers, in collaboration with the Lung Cancer Mutation Consortium, have developed a process to analyze mutated genes in lung adenocarcinoma to help better select personalized treatment options for patients. Adenocarcinoma is the most common type of lung cancer in the United States with approximately 130,000 people diagnosed each year.
The study, published in the May 21 issue of The Journal of the American Medical Association, investigated 10 highly mutated and altered genes that contribute to cancer progression, termed oncogenic driver genes, in more than 1,000 lung cancer patients. Patients with adenocarcinoma have a high probability of having mutated oncogenic driver genes in their tumors.
As new oncogenic driver genes are identified, the testing process becomes less efficient because more genes need to be analyzed in limited amounts of tumor tissue. The researchers developed a process to analyze multiple genes at one time with small amounts of patient tissue. They found that 64 percent of lung adenocarcinoma patients had at least one oncogenic driver gene.
Patients who had mutations were offered therapies targeted to their specific mutation. The researchers found that those patients who had targeted treatment against an oncogenic driver gene survived longer than those patients who did not.
"Precision medicine is the future of cancer care. We are continuing this study by attempting to profile all advanced lung adenocarcinoma patients for driver genes to match them with appropriate therapies," explained Eric B. Haura, M.D., director of Moffitt's Lung Cancer Center of Excellence. "We'd like to extend this further to examine for driver genes in other types of lung cancer, such as squamous cell lung cancer."
The researchers also plan to use this technology to study drug resistance and are developing additional platforms to guide decision making in the clinic.
Since the Lung Cancer Mutation Consortium trial began in 2009, many patients are now tested for mutated or altered genes before treatment. This study is the first of its kind that supports the concept that the simultaneous analysis of genetic mutations is possible with the goal of delivering more personalized medicine.
More information: jama.jamanetwork.com/article.a … px?articleid=1872815
Journal information: Journal of the American Medical Association