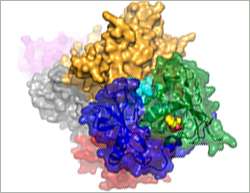
Crystal structure of thalidomide (yellow calotte model) bound to CRBN (green, blue and cyan) and DDB1 (yellow, grey, red and purple).
(Medical Xpress)—Scientists led by Nicolas Thomä at the Friedrich Miescher Institute for Biomedical Research have clarified the workings of thalidomide at the molecular level. Their analysis of various structures, published in Nature, indicates that the drug can interfere with cellular processes in two different ways—once preventing and once promoting protein degradation—thus explaining its diverse clinical effects.
In the early 1960s, thalidomide – a drug widely prescribed at that time as a sedative and for the treatment of morning sickness in pregnancy – became notorious when it was found to cause birth defects. The product was subsequently withdrawn from the market. In the late 1990s, however, thalidomide and its derivatives were shown to be effective in the treatment of certain types of blood cancer, and the drug has come back into use – subject to strict safety requirements.
In a study published in Nature, Nicolas Thomä and his group of structural biologists at the Friedrich Miescher Institute for Biomedical Research have now revealed how thalidomide interacts with its target protein in the cell, giving rise to the different kinds of clinical effects.
Thalidomide has been known to interact with a large protein complex known as DDB1-CRBN. DDB1-CRBN is part of a major protein degradation system, which uses the molecular tag ubiquitin to mark various proteins for degradation. Such complexes are also known as E3 ubiquitin ligases.
With the aid of crystal structures of thalidomide and its derivatives bound to DDB1-CRBN, the scientists found that, depending on cell type and configuration, thalidomide either activates or inhibits DDB1-CRBN. In the first case, which is relevant to the treatment of blood cancer, thalidomide promotes the attachment of ubiquitin to two proteins known as Ikaros and Aiolos. Thalidomide recruits these two transcription factors to the realm of the ubiquitin ligase action.
In the second case, thalidomide blocks a normal substrate from binding to DDB1-CRBN. The binding partner in question – previously unknown – was identified as MEIS2, a protein involved in various aspects of human development. Thalidomide thus prevents MEIS2 from binding to DDB1-CRBN and degradation.
Thomä comments: "We're convinced that our findings provide initial evidence of how thalidomide and its derivatives can lead to both beneficial and extremely adverse clinical effects. Only with experiments like these will we be able in the future to produce compounds specifically designed to enhance the benefits and minimize the adverse effects."
More information: Fischer ES, Böhm K, Lydeard JR, Yang H, Stadler MB, Cavadini S, Nagel J, Serluca F, Acker V, Lingaraju GM, Tichkule RB, Schebesta M, Forrester WC, Schirle M, Hassiepen U, Ottl J, Hild M, Beckwith REJ, Harper JW, Jenkins JL, Thomä NH (2014) (2014) "Structure of the DDB1 CRBN E3 ubiquitin ligase in complex with thalidomide." Nature. 2014 July 16 DOI: 10.1038/nature13527. [Epub ahead of print]
Journal information: Nature