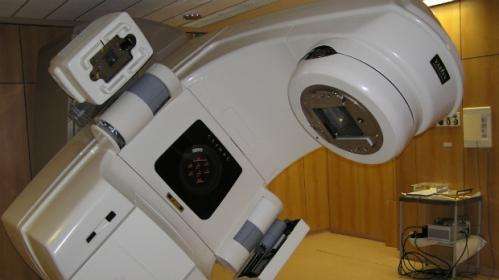
In new draft guidance, The National Institute for Health and Care excellence (NICE) is set to recommend a new form of radiotherapy for people with early stage breast cancer.
The treatment, known as Intrabeam Radiotherapy, can be given during surgery instead of as a course of treatment. A clinical trial in 2013 suggested it was likely to be as effective as conventional radiotherapy.
However it is likely to be some time before clinical staff have been trained to use the technique, and that all treatment centres have access to the equipment needed to deliver it.
Professor Carole Longson, NICE's director of health technology evaluation at NICE, said: "Unlike regular radiotherapy, with the Intrabeam Radiotherapy System only one dose is required.
"This single dose is given at the same time as surgery, eliminating the need for numerous hospital visits.
"Regular radiotherapy typically requires numerous doses over a three week period - although some people may receive it for longer - and is performed weeks or months after surgery or chemotherapy."
Emma Greenwood, Cancer Research UK's head of policy said the announcement could be "good news" for breast cancer patients.
"Giving radiotherapy in a single dose at the time of surgery potentially offers a huge benefit, especially if it means fewer visits to hospital," she added.
"Radiotherapy is already a very effective treatment, and this technique offers another valuable option for treating early breast cancer."
But NICE said that because the technique was new, it was recommending its use in a "controlled way", and that patients should be fully informed about the pros and cons of treatment.
"It's still a new treatment - so far only six centres in the UK have used the Intrabeam Radiotherapy System to treat early breast cancer," added Longson.
And since the treatment is so new, doctors would need to gather more evidence about the treatment's long term effectiveness, she said.
Cancer Research UK's Emma Greenwood agreed. "It's essential that those who receive this radiotherapy are followed up for a long period of time to ensure the single dose is as at least as effective as the standard treatment," she said.
Every year, around 41,500 women and 300 men in England are diagnosed with breast cancer.
NICE said that around 86 per cent of these patients, or 35,970 people each year, could potentially benefit from the treatment.
The guidance is now open for consultation until August 15, with a final recommendation expected later this year.
A Intrabeam radiotherapy machine costs £435,000 plus VAT and maintenance of the machine is expected to cost in the region of £35,000 a year.
More information: The consultation documents are available online: www.nice.org.uk/Guidance/InDevelopment/GID-TAG353
Provided by Cancer Research UK