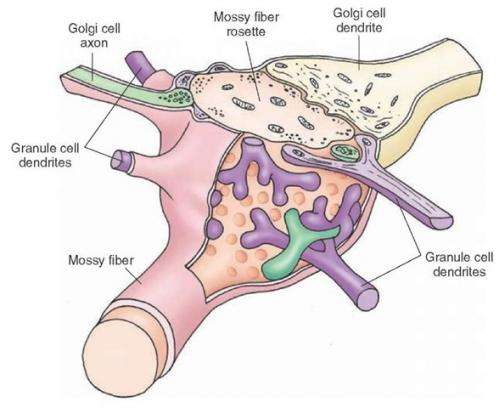
Mossy fiber synaptic rosette. Credit: what-when-how.com
(Medical Xpress)—One of the challenges in high energy physics is to understand the origin of cosmic rays. The problem is that although these rays continue to be observed at ever higher energies, there is currently no known physical mechanism to fully explain how they attain that energy. The same issue is found in neuroscience. Researchers continue to find neurons that fire spikes at higher and higher rates (>1500 hz) but the models that traditionally have been used to describe them start to break down far below that.
The so-called mossy fibers of the cerebellum are one part of the brain where in vivo recordings have recently been breaking speed records. Mossy fibers originate outside the cerebellum, typically from the pontine nuclei, vestibular nerve, and reticular formation. The unique thing about these myelinated axons is that it doesn't seem to matter where they originate from, once in the cerebellum they do as cerebellar afferent fibers do. For those contacting the deep layer granule cells, this means forming large synaptic boutons as they wind through fawning agglomerations of dendrites, giving them a mossy appearance.
Granule cells are by far the most numerous cells in the entire brain, one-hundred billion of them by some estimates. The cerebellar mossy fiber boutons (cMFBs) are highly divergent structures and each attracts dendrites from over ten granule cells. A group of researchers in Germany decided to look at in vitro slices of the cerebellum to see just how fast they could push the mossy fiber machinery. By using a technique called two-photon targeted patch clamping they could find individual cMFBs for stimulation and recording. They could also simultaneously patch onto the granule cell connections and determine the cut-off point at which transmission across the bouton could no longer track the input.
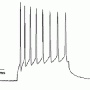
The team, led by corresponding author Stefan Hallermann, reported their results in the latest issue of Neuron. By stimulating mossy fibers with 20 depolarizing pulses they determing that action potentials could follow at rates up to 1600 hz before dropping a spike from the train. The temporal width of the generated spikes at the point of half of their maximum amplitude was only 100us, and that tightness was maintained up through the 1600 hz limit before broadening slightly. 100us is half that of anything previously reported and the authors attributed this astounding repolarization speed to the Kv1 and Kv3 potassium channel subtypes they found in the mossy fibers.
We previously reported on studies that examined what happens when synapses run out of transmitter. Those studies stimulated gabaergic hippocample neurons at a much more modest 4 hz, and invariably found significant transmitter rundown after 2000 or so pulses. For stimulation above a kHz, a synapse would need pretty fast vesicle filling and recycling to maintain reliable transmission for any length of time. However once we are talking about the kHz regime, there are more pressing issue before one even gets to the transmitter logistics. In particular, there is the problem of recruiting the vesicles to the so-called releasable pool in the first place.
The researchers found that the cMFBs showed reliable transmission to up to 12 granule cell partners at a time. By careful measurements of synaptic currents and capacitance changes, they estimated the vesicle recruitment rate was around 400 vesicles/sec at each release site, and a colossal 30,000 vesicles/sec for the entire bouton. This is at least 3 times greater than that observed at other high speed synapses such as the calyx of Held synapse in the auditory system. The authors note here that maintaining smooth flow from spike to transmitter release at these speeds requires both tight coupling of vesicles to Ca2+ channels, and efficient opening kinetics for those channels.
To examine whether high speed kinetics or extreme density of Ca2+ and Na channels would be consistent with the short spike widths that were measured, the researchers turned to modeling. Their Hodgkin-Huxely model based on measured gating kinetics predicted spikes with half-durations much longer than those actually measured. A recent study of spike collisions has suggested alternative models which might explain spike propagation in terms of mechanical pulses. One challenge for these "soliton-like" spike models is that the neurotoxin TTX (which blocks Na channels with precise stoichiometry) clearly abolishes spikes at just .1nM concentrations. Early researchers curiously found that spikes could still be obtained without sodium or other monovalent ions in the solution, and also that excitability is still altered by TTX in the absence of Na.
A problem with using these older observations to bolster newer models is that there is a lot of nonspecificity floating around the ion channel world. I was discussing solitons with biphysicist Luca Turin who noted, for example, that Ca2+ also can pass through Na channels and Ca2+ currents can be blocked by TTX itself. Now that researchers have ways to actually see and probe the mechanical structure of tissue on small scales it makes sense to model the propagation of mechanical disturbances through them. One area where the physical structure of utmost importance is in myelin. As far as the absolute longitudinal speed of the spike itself we often hear that mammals, with their 100m/s pulses down axons with 50 turns of spiral myelin, are king. However, the Panaeid shrimp can actually send spikes twice that speed using a novel type of concentric myelin to augment their axons. The myelin in this creature does not hug the axon but rather retains a large submyelinic space, perhaps calling to mind for some the clear insulation layer in standard BNC cable.
The mossy fibers generate cMFB synapses that are actually more like full blown glomeruli. At 100um in diameter, it is the size of a large cell. On top of the granule cell dendrites there is usually another layer of golgi cell synapses, followed an investment of astocytic ensheathing processes. As yet there is no standard "glomerular" logic that gives order to this kind of synaptic mini-onion. In some places, like the olfactory bulb, the central member may be a dendrite rather than an axon, and the junction may have a clear direction and orientation. The question of who is penetrating who and to what end is often ambiguous in the heart of the glomerulus. While the flow of metabolic precursors might generally proceed from the outside layer towards the center, the flow of information in terms of vesicles might be imagined otherwise.
Electron Micrograph reveals dendrites in cerebellum
By any measure the cerebellum is puzzling structure. While it is possible for humans to amble through life without one, taking possession of our most incredible physical talents apparently requires one. The eyewire project has begun to map in detail the synaptic structure of the cerebellum. The video above show a reconstruction of electron micrographs where the individual vesicles and mitochondria can be captured in their totality. Studies, like the one described here, that reveal the upper bounds of synaptic flow are essential if we are to capture the fuller dynamic picture we can now only imagine.
More information: Ultrafast Action Potentials Mediate Kilohertz Signaling at a Central Synapse, Neuron, dx.doi.org/10.1016/j.neuron.2014.08.036
Journal information: Neuron
© 2014 Medical Xpress