In-depth analysis of bat influenza viruses concludes they pose low risk to humans
Zoonosis—transmission of infections from other vertebrates to humans—causes regular and sometimes serious disease outbreaks. Bats are a well-known vertebrate reservoir of viruses like rabies and Ebola. Recent discovery of sequences in bats that are resemble influenza virus genes raised the question of whether bat flu viruses exist and could pose a threat to humans. A study published on October 2nd in PLOS Pathogens addresses this question based on detailed molecular and virological characterization.
Because no infectious virus particles were isolated from the bat samples in which the influenza-like sequences were identified, the authors of the present study, led by David Wentworth from the J. Craig Venter Institute in Rockville, USA, and Wenjun Ma from Kansas State University in Manhattan, USA, first addressed the question of whether the sequences are derived from infectious flu viruses or merely evolutionary relics of now defunct ancestors.
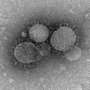
Using the published sequence information, they synthesized the entire genome (which consists of 8 genes) of a possible bat virus whose sequence was identified in 2009 (which they called Bat09) and put it into human tissue culture cells to see whether the viral sequences could (like human influenza viruses routinely do) hijack the cell's resources and machinery to manufacture bat flu viruses. It turned out they could: thousands of spherical influenza-like virus particles budded from the human cells into the culture medium. However, when they tried to infect a range of cells from different vertebrate species (including dog, pig, mink, monkey, human, and bats) with the Bat09 particles, they were unable to infect any of them.
Two surface influenza proteins, hemagglutinin (HA) and neuraminidase (NA), are located on the viral surface and known to be necessary for viral entry into host cells and viral particle release. Because bat influenza HA and NA are quite different from those of other flu viruses, the researchers replaced the two genes with those from another laboratory-adapted human flu virus called PR8 that can infect and multiply in a range of vertebrate cells, and also in chicken eggs. After they made sure that only the gene sequences that code for the HA and NA proteins but not the adjacent ones that direct packaging of the gene into new bat flu viral particles were switched, they found that this hybrid genome produced viruses that could infect and multiply in different vertebrate cells and in eggs.
Like the parental PR8 virus, the hybrid virus could also infect mice and cause serious flu-like illness. However, this seemed to be caused mostly by the PR8-derived HA and NA genes, because when the researchers created hybrids like before between Bat09 and a swine flu virus called TX98 that can infect mice but doesn't cause disease, the hybrid viruses behaved similar to TX98. When they tested some of the 6 genes who were still from Bat09, they could show that they function very similar to their counterparts from other (non-bat) flu viruses. One of them, for example, bat NS1, is a potent suppressor of the host immune response. The toxicity to mice of a second one, called PB2, could be manipulated by very small changes that made bat PB2 more like known very toxic or less toxic variants.
Reassortment (the random combination of genome parts from two or more different flu viruses that have co-infected a host cell) is responsible for most flu pandemics, because humans have little or no immunity against some of these new reassorted viruses. Based on several different tests for Bat09's ability to reassort with flu viruses from other species, it appears that bat influenza virus has very limited genetic and protein compatibility with Type A or Type B influenza viruses and may actually be a new type of influenza virus, which makes reassortment highly unlikely if not impossible.
"Collectively", the researchers conclude, "while our data suggest that the bat influenza viruses are authentic viruses and provide new insights into the evolution and basic biology of influenza viruses, the results also indicate that they pose little, if any, pandemic threat to humans."
More information: PLOS Pathogens , dx.plos.org/10.1371/journal.ppat.1004420