Neurotransmitter binding-site function revealed with unprecedented accuracy
What does it take for a drug molecule to turn on a protein? Sometimes nothing more than jostling a few atoms. New research from University at Buffalo scientists will help pharmacologists better understand how drugs work and how to make them more effective.
The study was published recently online before print in the Proceedings of the National Academy of Sciences.
"This research represents a significant advance in our understanding of how drugs activate receptors," explains Anthony L. Auerbach, PhD, professor in the Department of Physiology and Biophysics in the UB School of Medicine and Biomedical Sciences and senior author. "It highlights that at least in the receptor we studied, the acetylcholine receptor (AChR), sometimes just one specific amino acid, or even one atom, is sufficient to determine potency."
When released from the neuron, the neurotransmitter acetylcholine acts on skeletal muscles to cause them to contract; a related receptor is critical for the "controlled beating" of the heart.
"We're taking the ligand-receptor interaction apart and finding out piece by piece what makes muscles twitch," says Auerbach. "We have taken this approach down to the level of single side chains and even atoms to understand how a net energy emerges from the interactions between a drug and its receptor."
To the researchers' knowledge, it is the first time that single ligand-binding-site energies have been measured in any receptor.
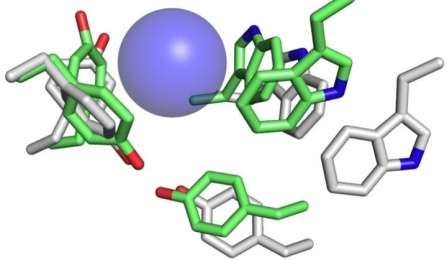
Auerbach's co-author, Tapan K. Nayak, PhD, a postdoctoral associate in the UB lab, and colleagues made novel energy measurements from eight different functional groups at three different kinds of neurotransmitter binding sites in fetal and adult type AChRs. The group is studying how specific functional groups in the AChR respond to acetylcholine and other drugs with similar effects.
They found that one specific amino acid present in the fetal-type, but not in adult organisms, contributes enormously to the action of acetylcholine and choline, to generate a greater potency. It was further studied through computer simulations done by the Center for Computational Research, UB's supercomputing center. The research is relevant to a birth-related genetic disease: the multiple pterygium syndrome (also known as Escobar syndrome) in which one of the essential subunits unique to the fetal-type AChR, malfunctions.
Since the transition of fetal-adult AChR is critical for synapse formation, the study also will enhance understanding of how cells communicate with one another during development, Auerbach adds.
The finding may also bring closer to reality "receptor engineering," in which receptors can be engineered to bind more (or less) potently with natural molecules and drugs. "Once we know the mechanism of exactly how a drug binds a specific molecule, we can engineer the receptor to be able to respond to it more effectively," Auerbach says.
The discovery was facilitated by the UB researchers' emphasis on measuring the energy changes that occur when chemicals bind to receptors. "Pharmaceutical industry researchers typically scan large random libraries of candidate molecules," he says. "What we've done is strategically pick the whole thing apart down to groups of atoms in the protein, and then predict their dynamic interactions with ligands. The key was to measure energies."
More information: "Functional differences between neurotransmitter binding sites of muscle acetylcholine receptors," PNAS 2014 111 (49) 17660-17665; published ahead of print November 24, 2014, DOI: 10.1073/pnas.1414378111