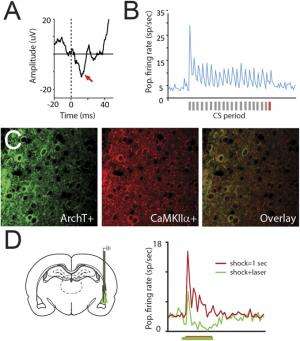
Auditory CS-evoked responding in LA cells, preferential ArchT expression in LA pyramidal neurons, and optical inhibition of aversive shock-evoked responding. (A) Population-averaged auditory-evoked field potential response amplitude (y axis) in response to the final auditory CS pip (the time point at which the auditory stimulus will overlap with the aversive shock during subsequent training) before threat conditioning. The x axis “0” point represents the onset of the auditory stimulus. Red arrows denote the short latency portion of the response, which is known to be potentiated following fear conditioning and was used for the statistical analyses as in prior work. (B) Population-averaged CS-evoked firing rate responses during the preconditioning test session from single tone-responsive LA neurons (n = 11/38 total cells) recorded in awake, behaving animals (−5 − 25 s total time period in PSTH from CS onset at first gray bar, 250-ms bins on x axis). Gray bars under the x axis denote individual auditory pips during the CS with the final pip denoted by a red bar. (C) ArchT (Left) and CaMKIIα (Center), a marker for LA pyramidal neurons, immunolabeling in LA sections. Overlayed image is shown on Right. (D, Left) Graphical depiction of dual optogenetic illumination and LA neural recording of shock-evoked responses. (Right) Population-averaged peri-event time histogram showing footshock-evoked firing rate responses (in spikes per second) in extracellularly recorded LA neurons (n = 7) without (red trace) or with (green trace) overlapping laser illumination. Shock-evoked responses were significantly larger during the shock alone compared with shock + laser trials (Wilcoxon matched-pairs test: Z = 2.20, P = 0.03). Credit: Johansen JP, et al. (2014) Hebbian and neuromodulatory mechanisms interact to trigger associative memory formation. Proc Natl Acad Sci USA 111(51):E5584-E5592.
(Medical Xpress)—In 1949, Donald O. Hebb (often called the father of neuropsychology and neural networks) published The Organization of Behavior: A Neuropsychological Theory1, connecting the two previously distinct areas of higher cognitive brain function and neural biology. His theory, known as Hebbian learning – and which came to be known as the Hebbian plasticity hypothesis – posited that "when an axon of cell A is near enough to excite a cell B and repeatedly or persistently takes part in firing it, some growth process or metabolic change takes place in one or both cells such that A's efficiency, as one of the cells firing B, is increased." (While often described in lay terms as cells that fire together, wire together, this omits the necessary causality involved: Hebbian learning requires that cell A fires just before, not coincident with, cell B – an important factor that presaged spike-timing-dependent plasticity, which adjusts the strength of connections between neurons in the brain based on the relative timing of a particular neuron's output and input action potentials, the latter referred to as spikes.)
That said, Hebbian plasticity is not the only theory that attempts to explain how memories are formed: some propose that neuromodulatory systems and Hebbian plasticity mechanisms must be co-activated to engage memory formation. (In neuromodulation, a neuron uses one or more neurotransmitters to regulate diverse populations of neurons, differs sharply from the form of synaptic transmission that Hebb focused on, where one presynaptic neuron, or cell A, directly influences a single postsynaptic neuron, cell B.) Recently, scientists at RIKEN Brain Science Institute, Japan and New York University presented in vivo evidence demonstrating that both Hebbian and neuromodulatory processes are necessary and sufficient to initiate synaptic strengthening and behavioral associative memory formation.
In a previous study2, the researchers employed optogenetics (a neuromodulation technique using lasers to control neurons genetically sensitized to light) to show that Hebbian mechanisms can cause aversive associative learning under artificial conditions having strong, iterative training. However, the current study found that under more moderate training conditions similar to those in daily life, while Hebbian mechanisms were necessary to produce neural plasticity in the lateral amygdala and thereby behavioral memory formation, alone they were not sufficient to generate these physiological and behavioral effects unless neuromodulatory systems were co-activated. The researchers suggest that this parallel process may be a general mechanism used by many of the brain's learning systems.
Joshua P. Johansen discussed the paper that he, Dr. Lorenzo Diaz-Mataix, Prof. Joseph E. LeDoux and their co-authors published in Proceedings of the National Academy of Sciences. Regarding their evidence of a co-activate parallel mechanism being necessary and sufficient to trigger synaptic strengthening and behavioral associative memory formation, Johansen says that in threat or fear conditioning, animals and humans learn that an auditory stimulus predicts the occurrence of an aversive outcome. "This occurs through strengthening of the connections between cells carrying auditory information and cells in the amygdala representing the aversive memory," he tells Medical Xpress. "The Hebbian hypothesis predicted that aversive experiences – for example, mild electrical shock – strongly activate amygdala neurons at the same time that an accompanying auditory cue activates weak inputs to the same neurons." This, Johansen explains, would then create a correlation in the electrical activity of the amygdala cells and the auditory neuronal connections with those neurons , resulting in an increase in the strength of connectivity between auditory and amygdala cells – and thereby the ability of the auditory system to then activate the fear system, producing these types of aversive memories.
"To test this idea," Johansen continues, "we needed a way to block the correlated activity which we showed occurred during the time that the tone and aversive shock were presented, and then measure the effects of this manipulation on the changes in the strength of the auditory connections with amygdala neurons and actual behavioral memory formation." To overcome this challenge, the scientists, as mentioned, used optogenetics to inhibit electrical activity in pyramidal neurons in the lateral nucleus of the amygdala during the aversive shock period. They then measured both the changes in the connections between auditory and amygdala cells and the behavioral memory. "This allowed us to block the correlated activity and directly test whether Hebbian mechanisms were necessary for the changes in these neural connections and the memory formation," Johansen notes. "However, this told us only that Hebbian mechanisms were necessary but not whether they were sufficient, making it possible that other factors that operated in parallel with these mechanisms were important in triggering memory formation."
Johansen points out that before the discovery of optogenetics, it was not possible to control electrical activity in specific populations of neurons with millisecond precision – and therefore it was not possible to manipulate the correlation in electrical activity between connected neurons to test the predictions of the Hebbian hypothesis and determine the effect of these manipulations on actual memory formation and the underlying changes in the neural connections. "We took advantage of the optogenetic approach," he says, "and used it in combination with behavioural and physiological techniques to overcome the technical limitations in testing the Hebbian hypothesis."
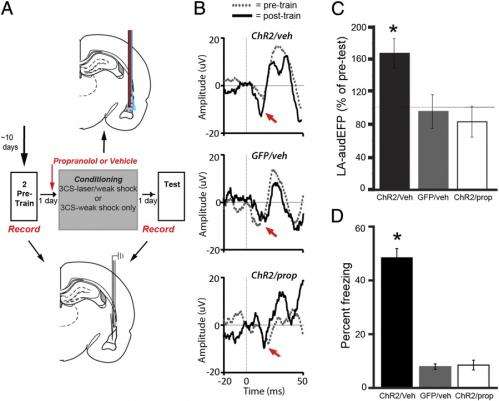
Hebbian and β-AR–mediated processes are required to trigger amygdala neural plasticity and aversive memories. (A) Experimental design for in vivo physiology study (Middle). Graphical depiction of LA propanolol/vehicle and laser delivery (Top) and physiological recordings before and after threat training (Bottom). (B) Combining optogenetic stimulation with weak shock potentiates the A-EFP in the LA in a β-AR–dependent manner. Sample traces of the amplitude of A-EFP responses for the ChR2/Veh group (Top), the GFP/Veh group (Middle) and the ChR2/prop group (Bottom) before (gray trace) and after (black trace) conditioning. Red arrows denote the short latency portion of the response that was used for the statistical analyses as in prior work. (C) Population-averaged A-EFP response pretraining vs. postraining [percent of pretraining baseline (y axis) in the ChR2/Veh group (black bar, n = 9), the GFP/Veh group (gray bar, n = 9), and the ChR2/prop group (white bar, n = 10). Gray line represents no change from baseline. (D) Percentage freezing during the LTM test in the same animals as in C. An asterisk indicates a statistically significant difference between the ChR2/Veh-treated group and both the GFP/veh and ChR2/prop groups. All error bars indicate SEM. Credit: Johansen JP, et al. (2014) Hebbian and neuromodulatory mechanisms interact to trigger associative memory formation. Proc Natl Acad Sci USA 111(51):E5584-E5592.
To examine this question the scientists tested whether Hebbian mechanisms on their own were sufficient to produce memories by eliminating the aversive outcome and using a different optogenetic approach to directly light stimulate electrical activity in the amygdala neurons while the auditory cue was present. This produced the correlation between the auditory connections with amygdala cells artificially in the absence of the aversive outcome. "We found that this did not produce fear memories unless many training trials were used, suggesting that Hebbian mechanisms were not sufficient on their own to produce aversive memories under normal conditions. However, if we stimulated neuromodulatory noradrenaline receptors in the amygdala at the same time we induced these artificial Hebbian processes it was possible to induce aversive memories." The researchers concluded that, considering their first set of experiments, this result indicated that both Hebbian and neuromodulatory mechanisms were acting in parallel in a synergistic way to trigger changes in auditory-to-amygdala connections to produce aversive memories.
To directly manipulate Hebbian mechanisms in the amygdala using optogenetics, the researchers needed to produce expression of optogenetic proteins – that is, proteins not normally expressed in the mammalian nervous system that respond to light – specifically in pyramidal neurons that project to other brain regions rather than in GABAergic cells, which inhibit neural activity only in cells within the amygdala. "We then needed to be able to manipulate these pyramidal cells with light while recording the electrical activity of amygdala neurons evoked by the auditory stimuli and behavioural memories," Johansen says. "To be able to express the optogenetic proteins specifically in pyramidal neurons, we took advantage of the fact that they express a protein called calcium⁄calmodulin-dependent protein kinase II alpha, or CaMKIIalpha – which GABAergic cells do not."
The researchers then made a DNA construct with the CaMKII promoter controlling the expression of opsins (light-sensitive proteins not normally expressed in the mammalian nervous system), packaged this DNA into a virus, and injected the virus into the amygdala. The virus would then infect the cells in the amygdala and the opsin proteins would only be expressed in the pyramidal neurons because the GABAergic cells lack the necessary elements to produce CaMKII-dependent protein expression. "Now that we had the amygdala pyramidal cells expressing opsins, we could proceed to our experiments."
The researchers then faced the challenge of optically regulating the electrical activity of pyramidal cells while those cells were expressing an excitatory or inhibitory opsin, and at the same time recording electrical activity from the amygdala neurons while the animals were learning. The scientists accomplished this by mounting a fiber optic cable alongside a metal recording electrode, surgically implanted alongside a fiber optic cable into the amygdala, and attaching the fiber optic cable to a laser.
In their paper, the scientists state that parallel neuromodulatory/Hebbian processes may represent a general mechanism used by many learning systems in the brain. "The amygdala participates in unconscious emotional memories of which sensory experiences produce unpleasant or pleasurable outcomes" Johansen says, "whereas other brain regions are responsible for other types of memories – such as those for places and events or about what behaviours lead to pleasurable or aversive outcomes – involving partially separate brain circuits that, like the amygdala for emotional memory formation, require changes in connectivity to produce memories. For example," he illustrates, "the hippocampus and associated cortical structures are responsible for memories of places and events, and the basal ganglia is involved memories related to linking behaviour with pleasurable or aversive experiences. This combined Hebbian and neuromodulatory mechanism we've elucidated in the amygdala may also be used by these other learning systems to produce neural changes underlying these other forms of memory."
Medical Xpress asked Johansen if, given that the central amygdalae nuclei are involved in the genesis of neuroendocrine responses, the hippocampus transcodes short-term memories to long-term memories, and the hypothalamus serves as an interface between the neural and hormonal systems, might these activities have any role in the results presented in the paper. "The type of fear/threat emotional memory we're measuring includes behavioral – cessation of movement or escape – and visceral – autonomic and hormonal – responses to sensory stimuli that predict danger" Johansen replied. "The lateral amygdala allows neutral sensory stimuli, such as tones, to become associated with aversive outcomes to produce these responses. The central amygdala is very important for neuroendocrine responses related to fear/threat learning as well as a number of the other fear/threat responses, including the one we've measured in our study – namely, behavioral freezing."
Johansen adds that the central nucleus of the amygdala receives input from the lateral amygdala and provides an output pathway to various other brain regions to produce the concerted fear/threat memory – and a projection of the central nucleus to the hypothalamus is responsible specifically for the hormonal aspects of the fear/threat response. "The hippocampus, which is involved in long-term conscious memories of events and locations, interacts with the amygdala system bidirectionally. Therefore, the amygdala connections with the hippocampus can provide emotional weight to these conscious memories and even enhance them, while in the other direction, the hippocampus connections with the amygdala can be associated with aversive outcomes much like simple sensory stimuli such as sounds and provide a way for locations and events to access this fear/threat system."
The paper also discusses the possibility that treatments for chronic pain and anxiety disorders may result from a deeper understanding of aversive instructive neural circuits such as those which are activated by aversive experiences and engage neuromodulatory and Hebbian processes to produce changes in connectivity within the amygdala. "Under conditions of chronic pain or anxiety disorders, these instructive circuits may be hyperactive and produce exaggerated aversive learning resulting in enhanced fear/threat memory formation, and ultimately psychiatric symptoms which accompany these disorders," Johansen explains. "This is a novel idea for how the psychological symptoms associated with chronic pain and anxiety disorders are produced that may motivate work in animal models of chronic pain or anxiety, and in human patients to determine whether these aversive instructive systems are dysregulated to produce the symptomology - and whether there are treatments which can resolve this dysregulation and improve patients' lives."
Moving forward, Johansen tells Medical Xpress that an important next step in their research is twofold – that is, determining which neural circuits generate these two signals, and why there are parallel systems rather than just a single system which initiates formation of these types of memories. "This will take us closer to understanding how the nervous system transduces aversive experiences into signals that produce memories. In addition," Johansen concludes, "with optogenetics rapidly advancing, we're interested in developing new optical approaches to manipulate brain circuits to better study these types of questions."
More information: Hebbian and neuromodulatory mechanisms interact to trigger associative memory formation, Proceedings of the National Academy of Sciences (2014) Published online before print, doi:10.1073/pnas.1421304111
Related:
1The Organization of Behavior: A Neuropsychological Theory, New York: Wiley and Sons (1949), New Edition (2002) ISBN-13 978-0805843002, eBook (2005)
2Optical activation of lateral amygdala pyramidal cells instructs associative fear learning, Proceedings of the National Academy of Sciences (2010) 107(28):12692-12697, doi:10.1073/pnas.1002418107
Journal information: Proceedings of the National Academy of Sciences
© 2014 Medical Xpress