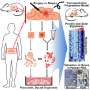
Long Island scientists have developed miniature pancreatic "organoids" that for the first time provide investigators with living three-dimensional models of the human gland to aid understanding of pancreatic cancer and ways to stop it.
Cancer of the pancreas is one of the most confounding diseases and rarely is caught at an early stage. Silent and lethal, only 7 percent of patients survive five years after diagnosis, doctors say.
With that in mind, researchers at Cold Spring Harbor Laboratory led by Dr. David Tuveson, embarked on creating cellular facsimiles of healthy and diseased human pancreatic tissue.
The organoids - produced from human pancreatic stem cells using complex tissue-engineering techniques - are transplanted into lab mice.
"When people see them (organoids) they remark how shiny they appear and how they look like little balloons in a sea of gelatin," said Tuveson, a professor at Cold Spring Harbor Lab and director of research for The Lustgarten Foundation in Bethpage. His research is financially supported by the foundation, established in 1998 in honor of former Cablevision vice chairman and Madison Square Garden chairman Marc Lustgarten before he died of pancreatic cancer. (Cablevision owns Newsday.)
Tuveson, his Cold Spring Harbor team and Dr. Hans Clevers, director of the Hubrecht Institute in the Netherlands, report their biological feat in Thursday's issue of the journal Cell. With organoids now in hand, the scientists say their work has the potential to change the way pancreatic cancer research is performed.
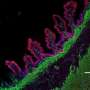
Up close, the organoids are small, spherical clusters of cells that can be easily seen through a microscope. But they can be propagated into a hefty enough size - although still small - to be seen with the unaided eye.
"They can be grown to be as large as a punctuation mark at the end of a sentence," added Tuveson, who is also on staff at Memorial Sloan-Kettering Cancer Center in Manhattan where he treats pancreatic cancer patients.
He said the organoids are derived from human pancreatic stem cells isolated from the pancreases of deceased people who donated their bodies to science. The pancreas is a 6-inch pear-shaped gland between the stomach and spine.
Stem cells are blank slates capable of morphing into complex tissues under certain laboratory conditions. Tuveson and his colleagues program the cells by plying them with growth factors. They do not achieve all of the complex cells of an adult pancreas, but focus on pancreatic ductal cells because those are most affected by the disease.
"We are growing simple structures that are hollow in the center," he said.
Cold Spring Harbor scientists now have models for each stage of pancreatic cancer, said cancer biologist Dr. Lindsey Baker, a postdoctoral fellow in Tuveson's lab.
She said 3-D organoids are truer representations of healthy and diseased pancreatic tissue than 2-D clusters of cells in a lab dish.
Baker added that when she and her colleagues transplant the organoids into mice, the cells assume the proper position, shape and function. Cancerous tissue advances through each disease stage, she said.
At the moment, doctors have been stymied in efforts to effectively treat pancreatic cancer.
"Pancreas cancer is the most lethal because we have the weakest medications available - and that's for any form of cancer," Tuveson said.
One hope for the outcome of the research, Baker noted, is ushering in an era of personalized medicine for pancreatic cancer. The Cold Spring Harbor team would like to see chemotherapeutic medications tested first on organoids derived from patients' cells, she said, to determine which work best.
The organoids, from the area of science known as regenerative biology, arrive amid a burgeoning number of Lilliputian body structures of all kinds.
Last week, scientists at Children's Hospital in Los Angeles announced the development of a 3-D organoid version of the human small intestine. That advance also was accomplished through the use of stem cells and tissue engineering.
Dr. Tracy Grikscheit said her work aims to treat children born with intestinal defects. The research has not yet advanced to a point where life-size portions of an intestine can be grown for surgical implantation.
Journal information: Cell
©2015 Newsday
Distributed by Tribune Content Agency, LLC