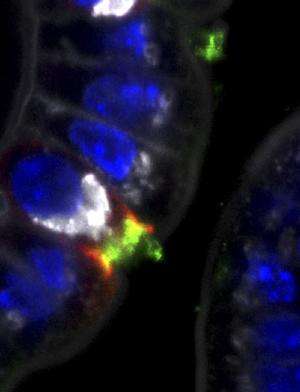
In this image of tissue from the intestinal wall of healthy mice, PI3-K is detectable (green) along with the presence of internalin A receptors (red). Credit: Gessain et al., 2015
A gut bacterium called Listeria (Listeria monocytogenes), which is often found in soft cheese, is known to present a risk to pregnant women. Listeria uses distinct tactics to breach the intestine and the placenta, using a protein called phosphoinositide-3 kinase (PI3-K), according to a study published in The Journal of Experimental Medicine.
Listeria has two proteins that help it cross mucosal tissue barriers. Both proteins, called internalins A and B, attach to tissue receptors and are needed for Listeria to invade the placenta, but protein A alone can propel Listeria across the intestine. What underlies these differences has remained unknown.
Tissue invasion by Listeria also requires the enzyme PI3-K. This enzyme is turned on by both of the Listeria's internalin proteins, but only the B protein has a built-in activation mechanism. Lecuit and colleagues at the Pasteur Institute in France have been able to visualize the activation of PI3-K, finding that this enzyme is very important for Listeria invasiveness via internalins. They uncover that PI3-K is perpetually turned on in intestinal cells, using only internalin A and rendering internalin B dispensable. The placenta, by contrast, has little to no inherent PI3-K activity, which is why passage of the bug through the placenta requires both A and B internalins.
These findings open up exciting new opportunities to examine whether other microbes—in addition to those posing a pregnancy risk—are capable of crossing host barriers using PI3-K activation, and whether this mechanism of bug invasion also occurs in other mucosal tissues and organs.
More information: Gessain, G., et al. 2015. J. Exp. Med. DOI: 10.1084/jem.20141406
Journal information: Journal of Experimental Medicine
Provided by Rockefeller University Press