The company you keep: Scientists reveal dual role for key T cell factor
When fighting chronic viral infections or cancers, a key division of the immune system, known as CD8 T cells, sometimes loses its ability to effectively fight foreign invaders. Overcoming so-called T cell exhaustion is crucial to treating persistent infections but the underlying molecular mechanisms remain poorly understood.
Now, a team of researchers at the La Jolla Institute for Allergy and Immunology report that the shift is masterminded in part by NFAT, short for Nuclear Factor of Activated T cells, and best known for its crucial role in getting CD8 T cells battle-ready. The findings from the lab of professors Patrick Hogan and Anjana Rao, Ph.D, published in the Feb. 17, 2015, issue of the journal Immunity, lay the groundwork for novel treatments to restore immune function.
"Understanding the molecular mechanism that leads to CD8 T cell exhaustion brings us a step closer to developing strategies to induce optimal T cell responses that can successfully clear infections and kill tumor cells," explains postdoctoral researcher and co-lead author Renata M. Pereira, Ph.D. "Conversely, it may allow us to interfere with autoimmune responses that paradoxically depend on the same protein".
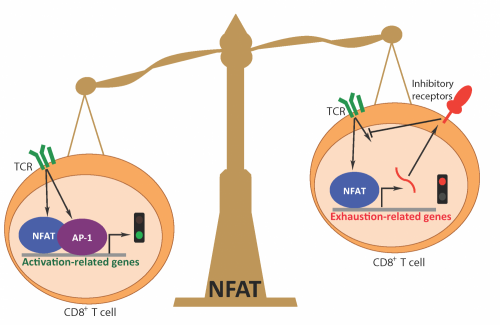
CD8 T cells are a subset of lymphocytes charged with killing cancer cells and cells that are infected with viruses or compromised in other ways. In previous work, the Rao and Hogan teams collaboratively pinpointed NFAT as the molecular hub that orchestrates T cell activation. When the T cell receptor on the surface of CD8 T cells recognizes a foreign protein, it kicks off a signaling cascade that culminates in the activation of NFAT and its partner AP-1. Together, the pair binds to regulatory regions in the genome and initiates a genetic program that activates T cells and readies them to fight cancer and viral infections.
In the face of chronic viral infections such as hepatitis and HIV as well as certain types of cancers, CD8 T cells become less effective over time until they ignore calls to arm. In addition, exhausted CD8 T cells start to express inhibitory cell surface receptors that receive and feed inhibitory signals into the cell establishing a negative feedback loop.
While a range of cellular markers of exhaustion, such as PD-1 and TIM3, have been characterized and are even the target of cancer immunotherapy drugs, the molecular details of how CD8 T cells switch gears were unclear.
Using NFAT as a starting point, Pereira and Gustavo J. Martinez, Ph.D., formerly a joint postdoc in the Rao and Hogan labs and now the Genomics Core Director at the Scripps Research Institute in Jupiter, Florida, established that interfering with NFAT's ability to partner with AP-1 tips the balance toward T cell exhaustion and and impairs the immune system's response to tumors and infections.
To gain a clearer picture of NFAT's role, the La Jolla Institute researchers embarked on a genome-wide survey of NFAT-binding sites in the genes occupied in activated versus exhausted CD8 T cells. The bioinformatics expertise of Professor Harri Lähdesmäki, Ph.D. and his graduate student Tarmo Äijö in the Department of Information and Computer Science at the Aalto University School of Science in Aalto, Finland was essential to this effort, said Rao.
Rao added that, "NFAT shifts the equilibrium between the activated state and exhaustion by binding to a different subset of regulatory regions within the genome." A closer look at the transcriptome—all the parts of the genome that are actively expressed at a given time—confirmed that NFAT, when acting on its own, induces a second transcriptional program that has many of the characteristic features of CD8 T cell exhaustion.
"Depending on the availability of AP-1, NFAT tips the scale toward T cell activation or exhaustion," says Martinez. In the presence of AP-1, NFAT induces T cell activation. Without it, NFAT initiates a negative regulatory program that activates genes encoding inhibitory cell surfaces markers and blunts signals received by the T cell receptor. It also interferes with CD8 T cells ability to produce cytokines, chemical messengers that recruit other arms of the immune system.
More information: "The transcription factor NFAT regulates exhaustion of activated CD8+ T cells" Gustavo J. Martinez, Renata M. Pereira, Tarmo Äijö, Edward Y. Kim, Francesco Marangoni, Matthew E. Pipkin, Susan Togher, Vigo Heissmeyer, Yi Chen Zhang, Shane Crotty, Edward D. Lamperti, K. Mark Ansel, Thorsten R. Mempel, Harri Lähdesmäki, Patrick G. Hogan, and Anjana Rao. Immunity, 2015. dx.doi.org/10.1016/j.immuni.2015.01.006