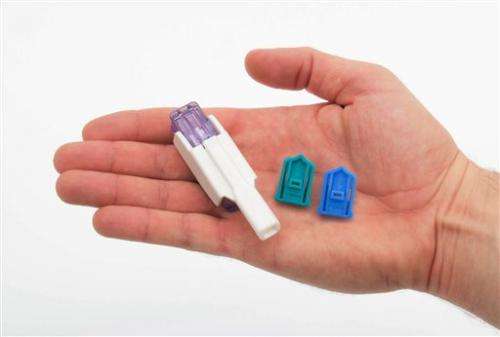
This photo provided by Sanofi shows an Afrezza inhaler with cartridges of inhalation powder. Hoping to appeal to millions of needle-phobic Americans with diabetes, drugmakers Sanofi and Mannkind have just launched Afrezza, an insulin that's inhaled, rather than injected. (AP Photo/Sanofi)
Hoping to appeal to millions of needle-phobic Americans with diabetes, drugmakers Sanofi and Mannkind have just launched Afrezza, an insulin that's inhaled, rather than injected.
Afrezza was approved by the Food and Drug Administration last June for patients with either Type 1 or 2 diabetes, to be used along with other medicines, diet and exercise.
Paris-based Sanofi SA, a major player in the huge diabetes treatment market, then licensed Afrezza's worldwide marketing rights from Mannkind Corp. of Valencia, California, which developed Afrezza.
"This is an alternative for those patients who are not willing to inject insulin" or to increase the number of shots per day, Stefan Schwarz, Sanofi's head of U.S. marketing for Afrezza, said in an interview Tuesday.
That's a lot of patients. More than 30 million Americans are estimated to have diabetes, an incurable hormonal disorder in which the body either doesn't properly respond to or doesn't produce enough insulin, which converts sugar from food into energy. As excess sugar accumulates, it damages blood vessels and vital organs and inhibits healing. The disease is a leading cause of amputations, blindness and other serious complications.
Meanwhile, a 2013 study in the journal "Diabetes Care" found that half of adults taking pills for diabetes delay the start of insulin use for five or more years to avoid injecting it in their stomach or elsewhere one or more times a day.
However, many patients don't have their diabetes under good control, and insulin usually does that better than pills.
Still, an inhaled insulin launched in 2006 by Pfizer Inc., Exubera, was pulled from the market in 2007 because of disappointing sales.
Schwarz noted that the formulation of Afrezza—different from Exubera's—disburses it widely in the lungs so it enters the bloodstream rapidly and hits peak levels in just 15 minutes.
"You can basically plan around your day, rather than planning around your insulin," Schwarz said.
By comparison, injected insulin peaks in the blood in about 90 minutes, so patients must inject it well before each meal and know in advance what and how much they'll be eating to calculate the dose.
Afrezza's most common side effects are low blood sugar, cough, throat pain or irritation and headaches. Because it can cause a tiny decrease in lung function initially, it's not recommended for patients with asthma, emphysema or chronic bronchitis or who smoke or recently stopped smoking.
Sanofi said its dry-powder insulin and inhaler have other advantages over Exubera: Afrezza's inhaler is much smaller, is replaced every 15 days so it doesn't need cleaning, and it dispenses insulin in "units" as with injected insulin, making dose calculations easy. Exubera's inhaler required metric system calculations.
Sanofi said the daily cost for Afrezza should range from about $7.50 to $9.25 per day, for typical doses, compared to about $3.10 to $5.20 for the company's injected insulin brand Aprida.
Sanofi, the world's third-biggest drugmaker by revenue, needs to boost its U.S. diabetes business. It's been slumping—one reason the company ousted CEO Chris Viehbacher last year—and the patent on its top-selling insulin brand, Lantus, expires this month.
Sanofi is to receive 65 percent of Afrezza profits, Mannkind the remaining 35 percent.
© 2015 The Associated Press. All rights reserved.