Small loop in human prion protein prevents chronic wasting disease
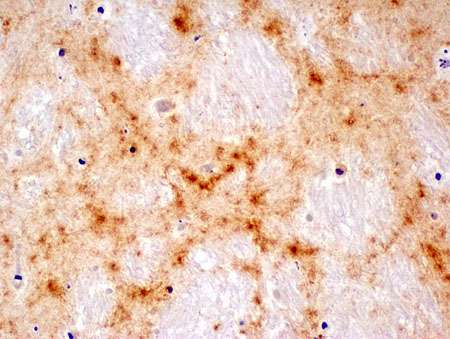
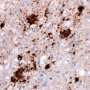
Chronic wasting disease (CWD)—an infectious disease caused by prions—affects North American elk and deer, but has not been observed in humans. Using a mouse model that expresses an altered form of the normal human prion protein, researchers at University of California, San Diego School of Medicine have determined why the human proteins aren't corrupted when exposed to the elk prions. Their study, published Feb. 23 in the Journal of Clinical Investigation, identifies a small loop in the human prion protein that confers resistance to chronic wasting disease.
"Since the loop has been found to be a key segment in prion protein aggregation, this site could be targeted for the development of new therapeutics designed to block prion conversion," said Christina Sigurdson, DVM, PhD, associate professor at UC San Diego and UC Davis and senior author of the study.
Prions aren't microorganisms like bacteria or viruses; they're simply protein aggregates. Some prion diseases are caused by an inherited genetic mutation, while others are caused by exposure to infectious prions in food. Acquired prion diseases are triggered when a foreign, misfolded prion protein causes the body's own natural prion proteins to misfold and aggregate. In addition to chronic wasting disease, examples include scrapie and bovine spongiform encephalopathy (or "mad cow disease") in animals and variant Creutzfeldt-Jakob disease in humans. In humans, prion diseases can cause a variety of rapidly progressive neurological symptoms, such as difficulty walking and speaking, and dementia. These diseases are 100 percent fatal and there is currently no effective treatment.
"We suspected that a loop in the human prion protein structure may block the elk prions from binding, as the sequences did not appear to be compatible," Sigurdson said.
To test this hypothesis, Sigurdson and her team developed a transgenic mouse that expresses a prion protein that's identical to the human version—except for a small loop, which they swapped out for the elk prion sequence. When these mice were exposed to the elk prions, they developed chronic wasting disease.
In contrast, control mice expressing the normal human prion sequence resisted infection when exposed to same materials—just as humans seem to, even those who consume venison meat.
"This finding suggests that the loop structure is crucial to prion conversion and that sequence compatibility with the host prion protein at this site is required for the transmission of certain prion diseases," Sigurdson said.