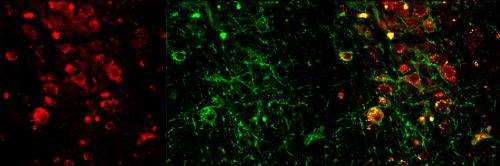
Confocal images of cells in an area of the brain stem called the PPT show expression of the neurotransmitter acetylcholine (red) and of a light responsive protein (green). The merged image on the right shows neurons expressing both. Credit: Christa Van Dort, PhD, Massachusetts General Hospital and Massachusetts Institute of Technology
Researchers at Massachusetts General Hospital (MGH) and Massachusetts Institute of Technology (MIT) have added another piece to the complex puzzle of how the brain controls one of the most essential functions - sleep. In their report in the January 13 issue of PNAS, they describe finding that activation of cholinergic neurons - those that release the neurotransmitter acetylcholine - in two brain stem structures was able to induce REM (rapid-eye movement) sleep in an animal model. Better understanding of brain mechanisms that control different sleep states is essential for the treatment of sleep disorders.
"Sleep is crucial for survival and maintenance of health, and sleep disorders impair many brain and body functions - such as complex thinking, the immune system and memory consolidation," says Christa Van Dort, PhD, of the MGH Department of Anesthesia, Critical Care and Pain Medicine and the MIT Department of Brain and Cognitive Sciences, lead author of the PNAS paper. "Natural sleep is composed of alternating cycles of non-REM and REM sleep, each of which provide different benefits. Current sleep aids do not effectively restore normal sleep physiology or timing and as a result do not fulfill the important functions of natural sleep. To develop new strategies for reproducing natural sleep, we need to understand the control of each component individually and in combination."
Several previous animal studies have suggested that cholinergic neurons in two areas of the brain stem - the laterodorsal tegementum (LDT) and the pedunculopontine tegmentum (PPT) - have important roles in REM sleep, but other investigations have had conflicting results. Both the LDT and PPT have populations of neurons that release neurotransmitters other than acetylcholine, some of which also are active during REM sleep. So to get a more precise picture of the role of cholinergic neurons in REM sleep, the investigators used optogenetics, a method of activating specific populations of neurons by means of light.
In experiments with mice genetically engineered to express a light-responsive protein in all cholinergic neurons, the researchers found that activation during non-REM sleep of cholinergic neurons in either the LDT or the PPT increased the probability that animals would enter REM sleep. LDT or PPT activation also increased the number of REM sleep episodes but not their duration, implying that different mechanisms underlie the initiation and the maintenance of REM sleep.
"Each stage of sleep provides different benefits - for example, deep non-REM sleep is associated with feeling rested, and REM sleep is important for learning - so improving our understanding of the areas of the brain that control each sleep state is an important first step toward being able to reproduce natural sleep patterns," says Van Dort, who is an instructor in Anesthesia at Harvard Medical School. "Now we are investigating how the cholinergic system connects with other brain regions important in non-REM sleep regulation as well."
"Christa's findings have important implications not only for understanding sleep, but also for understanding how the brain controls arousal level in general," says senior author Emery Brown, MD, PhD, of the MGH Department of Anesthesia, Critical Care and Pain Medicine, who is the Warren Zapol Professor of Anaesthesia at Harvard Medical School and the Taplin Professor of Medical Engineering at MIT. Support for the study includes National Institutes of Health grants DP1-OD003646, TR01-GM104948 and T32-HL07901.
Journal information: Proceedings of the National Academy of Sciences
Provided by Massachusetts General Hospital