Study bolsters 'turbocharged' protein as a promising tool in hemophilia gene therapy
Using gene therapy to produce a mutant human protein with unusually high blood-clotting power, scientists have successfully treated dogs with the bleeding disorder hemophilia, without triggering an unwanted immune response. In addition, the "turbocharged" clotting factor protein eliminated pre-existing antibodies that often weaken conventional treatments for people with hemophilia.
"Our findings may provide a new approach to gene therapy for hemophilia and perhaps other genetic diseases that have similar complications from inhibiting antibodies," said the study leader, Valder R. Arruda, M.D., Ph.D., a hematology researcher at The Children's Hospital of Philadelphia (CHOP).
Arruda and colleagues published their animal study results in the print edition of Blood on March 5.
Hemophilia is an inherited bleeding disorder that famously affected European royal families descended from Queen Victoria. Most commonly occurring in two types, hemophilia A and hemophilia B, the disease impairs the blood's ability to clot, sometimes fatally. When not fatal, severe hemophilia causes painful, often disabling internal bleeding and joint damage.
Doctors treat hemophilia with frequent intravenous infusions of blood clotting proteins called clotting factors, but these treatments are expensive and time-consuming. Moreover, some patients develop inhibiting antibodies that negate the effectiveness of the infusions.
For more than two decades, many research teams, including at CHOP, have investigated gene therapy strategies that deliver DNA sequences carrying genetic code to produce clotting factor in patients. However, this approach has been frustrated by the body's immune response against vectors—the non-disease-causing viruses used to carry the DNA. Those responses, which defeated initial benefits seen in experimental human gene therapy, were dose-dependent: higher amounts of vectors caused more powerful immune responses.
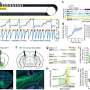
Arruda and colleagues therefore investigated gene therapy that used lower dosages of vector (adeno-associated viral-8 vector, or AAV-8 vector) to produce a more potent clotting factor—a variant protein called FIX-Padua.
Arruda was part of a scientific team in 2009 that discovered FIX-Padua in a young Italian man who had thrombosis, excessive clotting that can dangerously obstruct blood vessels. A mutation produced the mutant clotting factor, called FIX-Padua, named after the patient's city of residence. This was the first mutation in the factor IX gene found to cause thrombosis. All previously discovered FIX mutations lead to hemophilia, the opposite of thrombosis.
FIX-Padua is hyperfunctional—it clots blood 8 to 12 times more strongly than normal, wild-type factor IX. In the current study, the researchers thus needed to strike a balance—to relieve severe hemophilia in dogs, by using a dose strong enough to allow clotting, but not enough to cause thrombosis or stimulate immune reactions. "Our ultimate goal is to translate this approach to humans," said Arruda, "by adapting this variant protein found in one patient to benefit other patients with the opposite disease."
The current study tested the safety of FIX-Padua in three dogs, all with naturally occurring types of hemophilia B very similar to that found in people. Two of the dogs had never been exposed to clotting factor, and had never developed antibodies. The gene therapy injections changed their hemophilia from severe to mild, with no bleeding episodes for up to two years. They did not develop inhibitory antibodies, nor was there evidence of thrombosis.
The third dog, named Wiley, already had inhibitory antibodies before receiving the gene therapy. Wiley also experienced safe and effective treatment of hemophilia, persisting over a sustained period— three years. The treatment also eradicated the inhibitory antibodies, the first time this occurred in an animal model with pre-existing antibodies.
Another set of preclinical safety studies in mice supported the safety and efficacy of gene therapy using FIX-Padua. Arruda added that larger studies are needed in dogs with pre-existing inhibitors, to confirm these encouraging early results.
In the meantime, at least one clinical trial is making use of FIX-Padua in adult patients with hemophilia B—at the University of North Carolina at Chapel Hill, under Paul Monahan, M.D. Leaders of a separate trial being planned at Spark Therapeutics in Philadelphia, under Katherine A. High, M.D., are contemplating using FIX-Padua as well.
More information: JM Crudele et al,"AAV liver expression of FIX-Padua prevents and eradicates FIX inhibitor without increasing thrombogenicity in hemophilia dogs and mice," Blood, published online Jan. 7, 2015 and in print March 5, 2015. doi.org/10.1182/blood-2014-07-588194