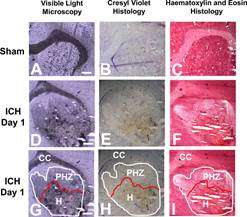
Visualizations of brain tissues help indicate the toxic iron pathways following haemorrhagic stroke. This data is vital to developing effective treatments for this severe type of stroke.
There's a stroke every 10 minutes in Canada. Of those, about 10-15 per cent are triggered by arterial ruptures and uncontrolled bleeding in the brain, and are incredibly devastating. These are the strokes that University of Saskatchewan researcher Dr. Mark Hackett studies, with hopes to help improve post-stroke health.
"Many people initially survive a stroke, but unfortunately a large percentage of the survivors retain long term damage" says Hackett.
Known as intracerebral haemorrhages, these strokes are a major health concern for Canadian men and woman of all ages. Brain damage is immediate and can continue in the hours, days and even weeks following a stroke. That damage can severely affect motor, logic, and verbal skills, and without immediate treatment, the brain can lose as many brain cells every hour as it does in about three and a half years of normal aging.
It is impossible to treat stroke immediately, even if you have a stroke in-hospital. That means that the best way to protect stroke victims is to understand the mechanisms behind damage after a stroke, and basic research is vital to developing therapies to reduce brain injury and improving stroke victims' quality of life, which is exactly the sort of data Hackett's team collects.
"We hope that by identifying mechanisms or harmful processes that are activated during intracerebral haemorrhage which then drive brain damage, we may be able to develop new therapeutic options for stroke patients. Such new therapies may help minimise or in some case prevent the loss of motor, logic, and verbal skills that occur after stroke" says Hackett.
In order to identify the pathways responsible for delayed brain cell damage, the team needed to precisely visualize chemicals in the brain.
Iron released into the brain from blood is thought to be a major contributing factor in chemical reactions that damage brain tissue after a haemorrhagic stroke. However, the brain has its own defense mechanisms that act to detoxify the iron released from blood, and being able to identify toxic and detoxified iron is key to developing effective stroke therapies.
"There are other techniques that you could use to detect harmful iron in the brain, but it would require dissecting a piece of tissue and grinding it up. Then information about exactly which brain cells have harmful iron is lost," says Hackett. So, Hackett developed a new synchrotron method to identify dangerous forms of iron in the brain.
Specifically, their new imaging method combines Raman spectroscopy, a technique commonly used for chemical analysis, and synchrotron-based X-ray Fluorescence Imaging, which can visualize the distribution of iron down to the cellular and even subcellular levels. Using this combination of techniques, it was possible for the team to not only identify where iron was present in the brain, but where and when it was dangerous to the brain's health.
The SMI team has now started new studies where they are using this suite of imaging tools to study how several potential therapeutic strategies may alter the amounts and location of toxic and non-toxic iron within the brain after intracerebral haemorrhage. They hope that this knowledge will then translate to the clinical setting for improved therapies for stroke victims.
More information: Hackett, M. J., Desouza, M., Caine, S., Bewer, B., Nichol, H., Paterson, P. G., & Colbourne, F. (2015). A new method to image heme-Fe, total Fe and aggregated protein levels after intra-cerebral hemorrhage. ACS Chemical Neuroscience. DOI: 10.1021/acschemneuro.5b00037
Journal information: ACS Chemical Neuroscience
Provided by Canadian Light Source


.jpg)

















