Cellular signals for pain fine tune neurons' sensitivity to opiods
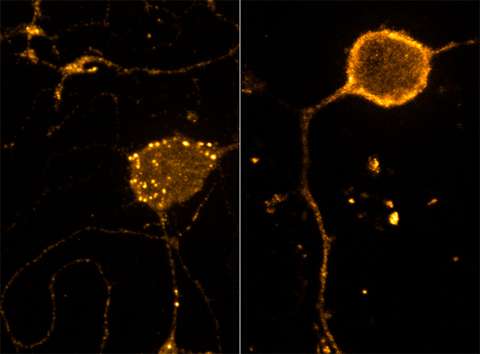
At the cellular level, pain and pain relief are caused by two different signaling pathways. But the two pathways aren't necessarily independent of one another, according to a study published by Carnegie Mellon researchers in Cell Reports.
The researchers determined the mechanism by which cellular signals for pain fine-tunes neurons' sensitivity to opioids, medications that relieve pain. The finding could help researchers better understand pain and addiction.
Pain management drugs represent a large market. Opioids, drugs that bind to opioid receptors on the surface of neurons, are effective against pain, but their use often leads to tolerance and addiction. Researchers have developed and studied more than 10,000 compounds based on morphine, but have yet to create a drug that has similar analgesic effects without causing addiction.
"Understanding how receptor localization changes in response to pain and medication is a different way to address the challenge of developing an non-addictive analgesic drug," said Manojkumar Puthenveedu, assistant professor of biological sciences and a member of the joint Carnegie Mellon and University of Pittsburgh Center for the Neural Basis of Cognition and Carnegie Mellon's BrainHub neuroscience initiative. "If we can control what happens to the receptor after the drug activates it, we might be able to better control how the body responds to opioids."
At the neuronal level, when a person experiences pain, a peptide called substance P activates the neurokinin 1 receptor on the neuron's surface. When a person takes an opioid to relieve pain, the drug activates a different receptor called the mu-opioid receptor. Once the receptors are activated, they begin a cascade of signals that cause or alleviate pain, and then get internalized into the cell. The mu-opioid receptor has long been a target for pain management drugs, but scientists haven't been able to figure out how to target the receptor in a way that alleviates pain but doesn't cause drug tolerance or addiction.
Puthenveedu and his colleagues wondered if perhaps crosstalk between the pain and analgesia signaling pathways might hold clues to this problem. Using a high-resolution fluorescence imaging technique Puthenveedu developed that allows researchers to visualize cell receptors in live cells and in real time, the researchers were able to show how pain signals change the rate at which mu-opioid receptors cycle from the cell membrane into the cell and back. When activated by DAMGO, an opioid that binds to the mu-opioid receptor and mimics endogenous opioids made in the body, only some of the receptors returned to the cell surface. This mechanism, in part, explains why patients quickly build tolerance to opioids; if the receptors aren't at the cell surface, the drugs can't bind to them and enter the cell.
The researchers in Puthenveedu's lab then used substance P to activate the pain-causing neurokinin 1 receptor in neurons that had been washed after DAMGO. By administering substance P, they accelerated the rate at which the mu-opioid receptors recycled back to the cell's surface, indicating that the mechanism could potentially play a role in combating tolerance to opioids.
They found similar results when they substituted fentanyl, a powerful opioid analgesic, for DAMGO. However, when they substituted morphine, pain did not increase receptor recycling. Puthenveedu says that this could help to explain to why endogenous opioids, fentanyl and morphine cause different responses in our body. In fact, when tolerance to these opioids was measured in a mouse model, substance P significantly reduced the development of tolerance to fentanyl, but not morphine.
More information: Cell Reports, dx.doi.org/10.1016/j.celrep.2015.02.045















