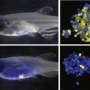
Dalhousie Medical School researchers have successfully been able transplant human leukemia cells into zebrafish embryos (shown above) to study their growth and test anti-cancer therapies. Credit: Nick Pearce photo
Two new cancer studies out of Dalhousie Medical School have shown success in testing safer, less toxic treatments for a rare form of pediatric leukemia called T-cell acute lymphocytic leukemia (T-ALL). The studies were published by first-year medical student, Victoria Bentley, and Drs. Graham Dellaire and Jason Berman, associate professors in the Departments of Pathology and Pediatrics, respectively.
"There are about 300 cases of childhood acute leukemia in Canada each year," says Dr. Dellaire. "80 per cent of those are acute lymphoblastic leukemia (ALL). T-ALL—specifically targets our body's T-cells and accounts for approximately 15 per cent of these cases."
"The studies are important because T-ALL is a high risk subset of leukemia that needs better therapies with less toxicities," says Bentley. "We need better ways to screen drugs before they enter clinical trials and better ways to design a patient's treatment plan. It's possible to do both with the zebrafish xenotransplant model."
The first study, published in Haematologica, demonstrates for the first time that T-cell leukemia from a patient's bone marrow can be successfully xenografted—where tissues of one species are transplanted to another—in zebrafish.
The process involves microinjecting a patient's tumor cells into the yolk sac of a 48-hour-old zebrafish embryo. As the zebrafish continues its natural growth, the T-ALL cells engraft in the zebrafish, allowing treatment response to be tested in the lab. An effective drug is identified when the xenografted cells in the zebrafish no longer divide—or ultimately die—compared to cells without a drug treatment.
"Our zebrafish xenograft model allowed us to determine the susceptibility of a patient's T-ALL within three days," says Dr. Dellaire. "If we were using this information to guide therapy, we could rapidly identify an effective drug and personalize a patient's therapy, reducing side-effects and improving outcome."
The co-authors are also using a zebrafish model to test protectants for cardiotoxicity: the damaging effects of chemotherapy on the heart.
Published in Science Translational Medicine, the group's second, high-impact study examined the chemotherapy drug doxorubicin, a common treatment for leukemias and many adult cancers, including breast cancer.
"When zebrafish embryos are treated in water with doxorubicin, they develop abnormalities of the heart," explains Dr. Berman. "Children treated with doxorubicin for leukemia often suffer heart damage, predisposing them to heart failure for many years following treatment."
The group's studies on chemoprotectants began through a collaboration with Dr. Randall Peterson at Massachusetts General Hospital and Harvard Medical School. The group identified a number of compounds that protects the heart against doxorubicin, the most promising being a drug called visnagin.
It was discovered that when visnagin was used in the group's leukemia zebrafish transplant model, the doxorubicin treatment retained it's ability to effectively kill the human leukemia cells xenografted in the zebrafish, but the visnagin prevented damage to the zebrafish heart.
"Clearly, if doxorubicin-like drugs are going to continue to be used, we need to be able to give these safely," says Dr. Berman. "Identifying new heart protective drugs is really important. Ultimately, drugs like visnagin may be combined with chemotherapy given to patients. The combination isn't planned for clinical trial yet, but is something that we hope to pursue in the future."
More information: "Visnagin protects against doxorubicin-induced cardiomyopathy through modulation of mitochondrial malate dehydrogenase." Sci Transl Med 10 December 2014: Vol. 6, Issue 266, p. 266ra170 DOI: 10.1126/scitranslmed.3010189
Journal information: Science Translational Medicine
Provided by Dalhousie University