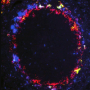
Schematic of a statistical model of antigen recognition by the adaptive immune system. After infection, antigen a encounters immune receptor r at random with a rate λa(t). An encounter leads to a successful recognition with a probability fr,a that reflects the matching between a given antigen–receptor pair. Credit: Andreas M, Balasubramanian V, Mora T, Walczak AM (2015) How a well-adapted immune system is organized. Proc Natl Acad Sci USA published online before print.
(Medical Xpress)—The adaptive immune system – a subsystem of the overall immune system – comprises specialized cells and processes that eliminate or prevent pathogen growth by using the experience of past infections to prepare its limited repertoire of specialized receptors to protect organisms from future threats. Recently, scientists at CNRS and Ecole Normale Superieure, Paris and the University of Pennsylvania developed a general theoretical framework from first principles that allowed them to predict the composition of receptor repertoires optimally adapted to minimize the biological cost of infections from a given pathogenic environment. Their theory predicts that the immune system will have more receptors for rare antigens; individuals exposed to the same infections will have largely different repertoires; and competitive antigen/receptor binding and selective amplification of stimulated receptors are key to creating optimal repertoires. Their findings explain how limited populations of immune receptors can self-organize to provide effective immunity against highly diverse pathogens, and moreover inform the design and interpretation of experiments surveying immune repertoires.
Researchers Thierry Mora and Aleksandra M. Walczak discussed the paper that they and their colleagues published in Proceedings of the National Academy of Sciences. "A great deal of very interesting theoretical work has been done on the problem of avoiding autoimmunity – that is, recognizing self-proteins – essentially viewing the immune system as a device for discrimination," Mora tells Medical Xpress. "We wanted to study the problem from a different perspective: As, in essence, introduced by Sir Frank Macfarlane Burnet's theory of clonal selection1, an adaptive immune system adapts to its pathogenic or antigenic environment. We wanted to see how far we could take the idea that the composition and diversity of the immune repertoire reflects that of the environment – in other words, the repertoire is an internal representation of its environment – aimed at minimizing the cost of infections to the tissues of the organism." In short, Mora says, this is one way to look at the complicated problem of the structure of immune repertoires.
This approach allowed the scientists to create a new framework that makes it unnecessary to explicitly model intracellular communication, cell differentiation, activation of cofactors, coordination of different cell types, the interaction with the innate immune system, and the full complexity of the recognition process. "Our goal was to make concrete predictions about adaptive immune repertoires that are general and not specific to one kind of cell type in specific conditions. While these features all play an important role in the functioning of real immune systems, we wanted to see what the essence was, what an optimal but simplified immune system would look like," Walczak points out. "We didn't want to concentrate on the fine details of repertoires, but rather took a step back and try to see what we could learn from global properties while still being realistic enough to make concrete statements about real immune systems – for example, the fact that two individuals in similar environments can have very different optimal immune repertoires." The scientists discovered that their assumption – that is, the system needs to minimize the cost of infection given a limited number of encounters – actually structures the repertoire.
At a high level, the scientists wanted their model to define an effective cost that could encompass and summarize all the aforementioned factors. They found that the surprisingly simple mathematical equation Fa(m) – a general cost function that measures the harm to an organism caused by non-recognition of a pathogen by the immune receptors associated with antigen a that have had m encounters with any pathogen. "Due to its phenomenological generality," Mora says, "there was no particular challenge in defining it – but we nonetheless considered several possible scenarios because there may be issues in deciding what its exact form and values should be." In short, Fa(m) describes how the harm effectively increases with the number of encounters.
"This is an approach that is common in physics, especially statistical physics," Walczak tells Medical Xpress, "where rather than describing the motion of all the particles that make up a gas, we write an effective equation of the state of the gas, or describe the change in the density distribution of the gas. In this study we do something similar: Since we know that there are many processes contributing to how harm increases with the number of infections, that it's impossible to describe them all, and that their detailed form does not really change the effect, we simply ask how we can describe their effect on the immune system. For example," she illustrates, "if the antigen population increases exponentially in real time – which seems like a sensible assumption seeing that non-inhibited pathogens will proliferate exponentially – and the harm to the organism increases with non-recognition also increases exponentially in real time. A simple calculation shows that this means Fa(m) will be linear in relation to the number of encounters."
At the same time, Walczak illustrates, it is easy to imagine that certain infections do not initially harm the organism, and/or that some do significant harm very quickly and then the harm saturates. In other words, one can imagine different costs – so because one can obtain the same effective cost from different molecular processes due to different instances of the infection, the researchers studied effective cost in different specific forms of this function.
"One of our predictions is that the optimal repertoires of two individuals sensing practically the same environment can be very different" Walczak continues. "The concrete position of the receptors in recognition space does not matter, as long as globally they tile antigenic space and provide good coverage. We showed that the immune system can find many optimal solutions to the same problem, so we should not be surprised if two individuals that live in the same conditions and are genetically close have very different repertoires." While these repertoires are optimal and thereby idealized, she notes, the scientists showed they are reached through traditional dynamics long considered when studying lymphocytes, or white blood cells in vertebrate immune systems.
"We also found that, in many situations, optimal repertoires cover the rare pathogens more thoroughly than we would have expected from just their frequency," Mora notes. "If a pathogen is, for example, 100 times more common than another, an adaptive immune system should probably not devote 100 times, but perhaps only 10 times, more resources."
The immune repertoire can self-organize to a state that minimizes cost and provides protection against infections via competitive evolution of receptor populations stimulated by antigens. Numerical simulations of the population dynamics, as well as its mean-field limit (Eq. 2), show how competition causes a random initial receptor distribution to fragment into a highly peaked pattern [Insets represent Pr(t) = Nr(t)/∑r ‘Nr’(t)]. Top Right Inset represents the antigenic environment Qa driving the dynamics [generated from a lognormal noise of power spectrum ∝1/(1 + (5q)2) and coefficient of variation 1]. Departure from optimality, as measured by the relative cost gap [<F>(Pr(T)) - <F>(P*r)]/ <F>(P*r), decreases with time and eventually reaches zero in the mean-field limit. The three independent runs of the stochastic dynamics show reproducible results. We use the availability function A(N) = 1/(1 + Ň/N0)2 with N0 =106, a death rate d = 0.001, and a cost function F(m) = 1 − e−βm with β =1/110. The space size is 10σ. The initial condition was drawn from a lognormal noise of power spectrum ∝1/(1 + (5q)2), with coefficient of variation 2 and ∑rNr(0) = 1.1 × 108. In the stochastic simulations, the time between antigen presentations is Δt = 0.005d−1 (200 infections per cell lifetime). Credit: Andreas M, Balasubramanian V, Mora T, Walczak AM (2015) How a well-adapted immune system is organized. Proc Natl Acad Sci USA published online before print.
The study's central finding is that limited populations of immune receptors can self-organize to provide effective immunity against highly diverse pathogens. "It's long been known that receptors and antigens are cross-reactive – that is, one receptor can recognize more than one pathogen and vice versa – which in principle allows the pathogenic space to be covered by a reasonably small number of receptors," Mora explains. "However, this does not have to be the case." Walczak notes that while the optimization problem they solve does not try to minimize the diversity of receptors, the optimal repertoires they found do have this limited diversity. "Cross-reactivity tells us that this is possible, but it being optimal surprised us."
The paper also reports that the fact that cross-reactivity (in which a receptor can bind to a variety of antigens) causes the optimal repertoire to fragment is related to the concept of limiting similarity due to competitive exclusion in ecological settings. "Cross reactivity eliminates the need for a unique receptor specific to each antigen – but only if the receptor can recognize the antigen," Walczak notes. "This means that different regions of the pathogen recognition space can have receptors and no receptors, respectively. In a way, it would be a waste to have receptors in places already covered by the cross-reactivity of other receptors." Their research demonstrates this by showing that tiling patterns emerge in the antigenic space as a result of repertoire dynamics in which receptors compete for antigens if one receptor "wins" another cannot be in the same place, a process similar to that found in ecology when species cannot live in the same niche. The organisms compete and one "wins," the other having to go elsewhere – a salient factor in speciation as the two groups evolutionarily diverge.
"Thanks to cross-reactivity," Mora adds, "it doesn't matter exactly where you put a receptor, as long as the entire antigenic space is covered – an observation related to stochastic hyperuniformity2,3 in disordered systems – that is, local randomness but global order."
The study's results follow from a tension between the statistics of pathogen detection, which favor a broader receptor distribution, and the effects of cross-reactivity, which tend to concentrate the optimal repertoire onto a few highly abundant clones. "There are two features that drive the form of the optimal repertoires," Mora explains. "On the one hand, it's important to protect yourself from the rare pathogens, not just the common ones - so you want to place receptors more or less evenly in recognition space. On the other hand, cross reactivity tells you that you do not have to put a receptor at every point of that space—you just have to space them out so there are no blind spots. It's these two properties that drive the form of the solution.
The paper also details the conceptual connection between the immune repertoire and ecological organization. "Since receptors divide and proliferate upon recognition of the antigens, the latter can be seen as resources on which the receptors thrive. Receptors recognizing the same antigens compete against each other – they belong to the same ecological niche, so to speak." Since each niche has limited capacity, only a few receptors may survive in each of them – a process known as competitive exclusion.
As described above, in order to achieve the tiling patterns of optimal repertoires receptors must compete for antigens. "One of the key results about optimal repertoires is that you want to protect yourself both from the rare antigens and the common ones – so resources must be distributed relatively evenly, depending on the details of how fast the cost grows with the number of unsuccessful encounters between receptors and antigen. Moreover, the system must keep the receptors that are good fits against common antigens from dominating. In terms of dynamics, this is achieved by a limited carrying capacity for each niche." Carrying capacity is the maximum population size of a given species that an environment's resources can sustain indefinitely without significantly depleting or degrading those resources.
Within this ecological context, the paper notes that living systems must often sense, internally represent, and respond to salient aspects of complex exogenous influences – and do so using limited resources, such as cell types or genes. For example, in the retina2 and the mammalian olfactory system3 the limited repertoire of resources constrains information processing, forcing these living systems to judicially parse resources in terms of priorities, costs and limitations in order to adapt to the environment. The scientists comment that they "have shown that these elements also shape the optimal form of the immune repertoire."
The scientists assumed that although the immune system cannot predict precisely which antigens it will encounter and when, it incorporates an estimate of the probabilities of their occurrences. "While the immune system cannot know with certainty when and what it will encounter, its goal is to protect us from the unknown," Walczak points out. "It's also very hard to characterize the set of pathogens because the space is just too big. However, if the set of potential threats was completely random from the point of view of the repertoire, the immune system would not be very efficient." In other words, the fact that the immune system is able to respond efficiently suggests that it has adapted to a specific environment, which in turn translates into it incorporating an estimate of the probabilities of the antigenic environment.
Moving forward, the scientists would like to measure recognition space by testing their predictions in high-throughput surveys of receptor and pathogen diversity. "In order to do this," Walczak comments, "we'd need to figure out how to map the sequence of receptors to recognition or affinity, or measure affinity in a high-throughput way." (Affinity measures the strength of interaction between an epitope – the part of an antigen recognized by the immune system – and an antibody's antigen binding site.)
The researchers expect that the new framework and their results will extend to other distributed protection systems where diverse threats are addressed by an array of specific responses. "Yes," Walczak agrees, "the framework is very general, which is why, as discussed, we see very similar properties in systems as diverse as ecology, neuroscience and the physics of disordered systems." In addition, the immune system of bacteria, or the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) system could be studied within a similar framework to predict the relative abundance of CRISPR spacers and corresponding viruses in a coevolving population of bacteria and viruses. "There's a tradeoff between a large number of threats/impulses and limited resources/processing abilities – and the immune system wants to minimize the chances of missing a threat/signal – and in our paper, she says, we've formalized this idea and made concrete predictions."
In addition to the connections with ecology, neuroscience and the physics of disordered systems discussed above, the study's results also inform the design and interpretation of experiments surveying immune repertoires. "There's a lot of interest in how different individuals – that is, either humans or genetically identical mice – respond to the same antigenic environments, and how different their natural repertoires are," Walczak says. "Our results show that it should not be surprising that the responses can be different, because even if their repertoires were optimal, two individuals would have different repertoires."
"Our predictions are about similarity in an effective recognition space," Mora concludes. "Most large scale probing of immune repertoires is based on sequencing the receptors – so mapping recognition space to receptor sequences is an important challenge for future experiments."
More information: How a well-adapted immune system is organized, Proceedings of the National Academy of Sciences (2015) published online before print, DOI:10.1073/pnas.1421827112
Related:
1A modification of Jerne's theory of antibody production using the concept of clonal selection, CA: A Cancer Journal for Clinicians (1976) 26(2):119–121, First published online 31 December 2008, DOI:10.3322/canjclin.26.2.119 (PDF)
2Local density fluctuations, hyperuniformity, and order metrics, Physical Review E (2003) 68:041113, DOI:10.1103/PhysRevE.68.041113 (PDF)
3Avian photoreceptor patterns represent a disordered hyperuniform solution to a multiscale packing problem, Physical Review E (2014) 89:022721, DOI:10.1103/PhysRevE.89.022721 (PDF)
4Eye smarter than scientists believed: Neural computations in circuits of the retina, Neuron (2010) 65(2):150–164, DOI:10.1016/j.neuron.2009.12.009 (PDF)
5A novel multigene family may encode odorant receptors: A molecular basis for odor recognition, Cell (1991) 65(1):175–187, DOI:10.1016/0092-8674(91)90418-X (PDF)
Journal information: Proceedings of the National Academy of Sciences , Physical Review E , Neuron , Cell
© 2015 Medical Xpress
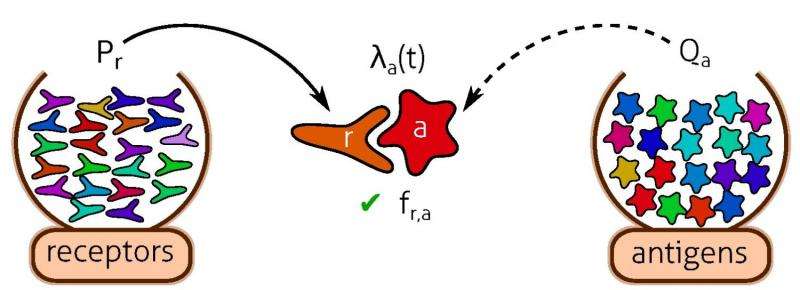
![The immune repertoire can self-organize to a state that minimizes cost and provides protection against infections via competitive evolution of receptor populations stimulated by antigens. Numerical simulations of the population dynamics, as well as its mean-field limit (Eq. 2), show how competition causes a random initial receptor distribution to fragment into a highly peaked pattern [Insets represent Pr(t) = Nr(t)/∑r ‘Nr’(t)]. Top Right Inset represents the antigenic environment Qa driving the dynamics [generated from a lognormal noise of power spectrum ∝1/(1 + (5q)2) and coefficient of variation 1]. Departure from optimality, as measured by the relative cost gap [<F>(Pr(T)) - <F>(P*r)]/ <F>(P*r), decreases with time and eventually reaches zero in the mean-field limit. The three independent runs of the stochastic dynamics show reproducible results. We use the availability function A(N) = 1/(1 + Ň/N0)2 with N0 =106, a death rate d = 0.001, and a cost function F(m) = 1 − e−βm with β =1/110. The space size is 10σ. The initial condition was drawn from a lognormal noise of power spectrum ∝1/(1 + (5q)2), with coefficient of variation 2 and ∑rNr(0) = 1.1 × 108. In the stochastic simulations, the time between antigen presentations is Δt = 0.005d−1 (200 infections per cell lifetime). Credit: Andreas M, Balasubramanian V, Mora T, Walczak AM (2015) How a well-adapted immune system is organized. Proc Natl Acad Sci USA published online before print. The immune repertoire can self-organize to a state that minimizes cost and provides protection against infections](https://scx1.b-cdn.net/csz/news/800a/2015/theimmunerep.jpg)