Asthma drug against dengue to be tested in clinical trial

A drug that has been used for over 30 years as an asthma and allergy medicine is now being tested to treat symptoms of dengue fever. The National University of Singapore (NUS) and Duke-NUS Graduate Medical School Singapore (Duke-NUS) are running a clinical trial, in collaboration with National University Hospital (NUH) and Singapore General Hospital (SGH), called KETODEN, to test the drug Ketotifen on patients who are infected with dengue.
Research that preceded the trial was done at Duke-NUS while the trial is being conducted in the Investigational Medical Unit (IMU) at NUS with patients from SGH and NUH.
Ketotifen is traditionally used to reduce the incidence of asthma and allergy attacks in patients. It works as an antihistamine and mast cell stabiliser. One of the symptoms of asthma is vascular leakage, or movement of fluid in the body that occurs when blood vessels are damaged. This is caused by the activation of a certain type of immune cell, the mast cell. Ketotifen works by blocking mast cells, which helps stop vascular leakage.
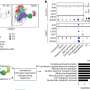
Assistant Professor Ashley St. John and her team, from the Duke-NUS Emerging Infectious Diseases Programme, were able to show that mast calls react strongly to dengue virus infection and release pro-inflammatory proteins, which may cause vascular leakage. It is hypothesised, that like in asthma, if Ketotifen can block mast cell activation in dengue, it may be able to block some of the more severe symptoms of dengue.
In dengue, mast cell activation leads to vascular leakage in animal models. Vascular leakage is the main complication of dengue where fluids from the blood are lost from blood vessels into other parts of the body such as the lining of the lungs. This reduces the overall blood volume and creates problems from excessive fluid build-up in areas such as the lungs or abdomen. Excessive or uncontrolled vascular leakage leads to dengue haemorrhagic fever or dengue with complications, in human patients.
The trial, led by Professor Paul Tambyah from the Department of Medicine at the NUS Yong Loo Lin School of Medicine, is aimed at determining if Ketotifen can safely alleviate these complications. Currently, dengue patients are often managed in outpatient clinics by providing fever and pain relief and advice on fluid intake. If vascular leakage is detected, or if patients have other complications, then they may be admitted for observation or intravenous fluids to prevent shock. However, there is no approved treatment to prevent vascular leakage during dengue infection.
Dengue fever is a mosquito-borne viral disease that is endemic in many parts of the world, including Singapore. Last year Singapore saw a rise in the number of dengue cases to almost 900 a week in peak months. This year, there have already been almost 3000 cases of dengue fever. Infection can lead to high fever, muscle aches, rash and vascular leakage. Vascular leakage is a key factor in the development of these more severe forms of the virus. There is currently no medicine approved for the treatment of dengue fever.
The collaborators of this trial at Duke-NUS, NUS, NUH and SGH are hopeful that Ketotifen will prove efficacious and prove to be an effective treatment for dengue, in a region that sorely needs one.