The stuffy noses and sinus pressure of head colds are uncomfortable, but for most people, they go away within days. For those with chronic sinusitis, however, those symptoms and others drag on for weeks. Now scientists are onto a potential new therapy that could address one of the underlying factors associated with the condition. They describe their work in the ACS journal Molecular Pharmaceutics.
In the body, nitric oxide (NO) plays a critical role in immunity. Researchers have found that this simple molecule is an important antimicrobial agent that helps prevent sinus infections. Low levels of NO in nasal passages have been linked to chronic sinusitis, a condition in which the sinuses become inflamed, making breathing through the nose difficult. It can also lead to facial pain and headaches. One therapeutic approach could involve boosting NO levels. Mark E. Meyerhoff and colleagues wanted to test out this idea.
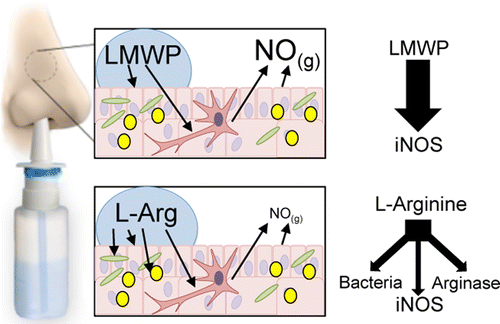
The researchers developed a simple method to make molecules called low-molecular-weight, arginine-rich peptides from an already-approved drug compound protamine. In lab tests, the peptides increased nitric oxide production in mouse immune cells and cells that line airways, making them a good candidate for further development.
More information: Enhancement of Inducible Nitric Oxide Synthase Activity by Low Molecular Weight Peptides Derived from Protamine: A Potential Therapy for Chronic Rhinosinusitis, Mol. Pharmaceutics, Article ASAP. DOI: 10.1021/acs.molpharmaceut.5b00110
Abstract
Nitric oxide (NO) is a key immune defense agent that is produced from l-arginine in the airways by leukocytes and airway epithelial cells, primarily via inducible nitric oxide synthase (iNOS). Deficiencies in nasal NO levels have been associated with diseases such as primary ciliary dyskinesia and chronic rhinosinusitis. Herein, we demonstrate a proof-of-concept regarding a potential new therapeutic approach for such disorders. We show that arginine-rich low molecular weight peptides (LMWPs) derived from the FDA-approved protamine (obtained from salmon sperm) are effective at significantly raising NO production in both RAW 264.7 mouse macrophage and LA4 mouse epithelial cell lines. LMWP is produced using a stable, easily produced immobilized thermolysin gel column followed by size-exclusion purification. Monomeric l-arginine induces concentration-dependent increases in NO production in stimulated RAW 264.7 and LA4 cells, as measured by stable nitrite in the cell media. In stimulated RAW 264.7 cells, LMWP significantly increases iNOS expression and total NO production 12–24 h post-treatment compared to cells given equivalent levels of monomeric l-arginine. For stimulated LA4 cells, LMWPs are effective in significantly increasing NO production compared to equivalent l-arginine monomer concentrations over 24 h but do not substantially enhance iNOS expression. The use of the arginase inhibitor S-boronoethyl-l-cysteine in combination with LMWPs results in even higher NO production by stimulated RAW 264.7 cells and LA4 cells. Increases in NO due to LMWPs, compared to l-arginine, occur only after 4 h, which may be due to iNOS elevation rather than increased substrate availability.
Journal information: Molecular Pharmaceutics
Provided by American Chemical Society