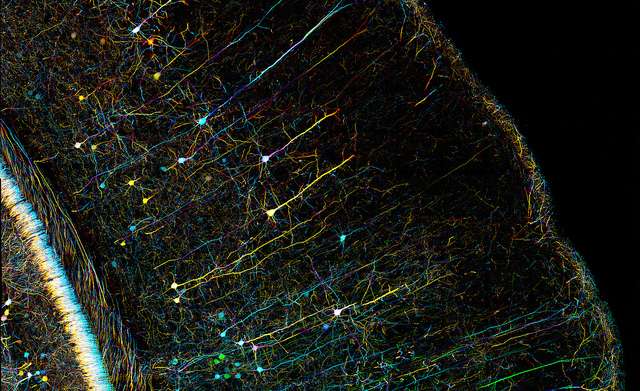
A microscopy image of a mouse brain. One of the major barriers to greater understanding of the biological mechanisms of mental illness is reliance on animal models, which have different experiences of mental illness than humans. Credit: Zeiss Microscopy
The human brain is capable of complex processes. The brain senses time and visualizes space. It allows us to communicate through language and create beautiful works of art. But what about when these cognitive abilities go awry? The National Institute of Mental Health (NIMH) cites serious mental illness (SMI) as a mental, behavioral, or emotional disorder that interferes with or limits one or more major life activities. The cited survey estimated the prevalence of SMI in the United States as ~4%, with the estimated prevalence for any mental illness being ~18%.
Mental illnesses, also known as psychiatric disorders, include many diverse conditions, including: anxiety disorders, Obsessive Compulsive Disorder (OCD), and post-traumatic stress disorder (PTSD). This group of illnesses presents a major global burden. In the 2010 Global Burden of Disease report, mental and substance use disorders comprised 7.4% of total disability-adjusted life years (DALYS) globally, and 8.6 million years of life lost (YLL), the single greatest cause of YLL worldwide. Psychiatric disorders also pose a significant burden to individuals and their families, and a challenge for clinicians and scientists.
For clinicians, many psychiatric disorders are difficult to treat. Even individuals with the same disorder present with a spectrum of symptoms and symptom severity, and many of the drugs currently used to treat these disorders have a multitude of undesirable side effects. For scientists, the mechanisms underlying this family of illnesses are still being unveiled, and reliable biological explanations for these disorders are still unclear, though it is known that biology and genetics play a role.
The lack of insight into the determinants of these disorders may relate to the difficulty in developing effective pharmacological treatments for them. Though various treatments for each of these disorders exist (ranging from drugs to cognitive behavioral therapy (CBT)), these treatments can be greatly improved. The field of neuroscience in particular is providing insight into the brain systems, cellular deficits, and genetics behind many of these disorders, which may help the development of new therapies. A limitation to current research efforts is that many of these insights come from the study of animal models. Also, conflicting results are often found in the literature. Despite these obstacles, the future of neuroscience holds a wealth of promise for developing a better understanding of psychiatric illness, studying these disorders with a new set of model systems, and interesting new research techniques.
Schizophrenia – An example of what we know
Perhaps one of the most studied of the psychiatric disorders is schizophrenia, a neurological disorder affecting about 1% of the general population and characterized by a variety of cognitive impairments, including a loss of affect and motivation, and often, the presence of hallucinations and delusions. A search in PubMed for articles with "schizophrenia" in the title yields more than 50,000 results, an indication of just how much research focuses on schizophrenia. Researchers have identified several hereditary factors (genes) with diverse sets of functions, that may be tied to this disorder..
- DISC 1 (disrupted in schizophrenia 1) is a gene that makes a protein with many interacting partners, and plays a role in a variety of pathways within cells—including the ability of cells to divide, mature, and move towards their final location within a tissue. Such processes have been shown to lead to neurological deficits and disorders if disturbed.
- Neuregulin 1 is a member of a protein family that has three other types of neuregulins. Perhaps most interestingly (and to make matters more complex), neuregulin 1 itself can also undergo a type of processing in the cell, called alternative splicing, that winds up producing many alternative forms of neuregulin 1 (up to 31 forms!) which all perform slightly different functions. The main job of neuregulin 1 seems to be to aid in brain and nervous system development.
- The CACNA1C gene is responsible for making a protein in the cell that forms part of an important calcium channel, playing a role in a variety of brain cell (neuron) function.
- Shank 3 is used by the cell as a scaffold, providing support for other cellular molecules that are important for the signaling that goes on between individual neurons of the brain.
Genes are not the only story though; researchers identified deficits at the cellular and network levels of the brain. The brain is comprised of both neurons and supporting cells called glia, the two major cell types of the brain. But there are a few classes of neurons (which change depending on the classification system you use), and each class is known to play its own important role in proper brain function. For instance, excitatory neurons use a chemical called glutamate to signal to other cells and are responsible for promoting the activity of their partners, whereas inhibitory neurons use a chemical called GABA to signal to their partners and are responsible for quieting these cells. There have been many reports of disrupted function of both excitatory and inhibitory neurons in mouse models of schizophrenia. These disruptions have been found in multiple brain regions and at different ages. But there have also been reports that fail to find a disruption of either of these cells types.
So what is the primary mechanism? This is exactly the problem outlined above: the complexity of mental illness makes it difficult for researchers to pin down a single biological explanation. Variations in mouse models, experimental approaches, animal age, or brain region being studied may be factors that contribute to inconsistencies across findings. The problem is that the brain changes over time and each brain region behaves a little differently from even its neighboring brain region. These factors complicate finding accurate and meaningful deficits in psychiatric disorders, a problem that may disappear in the near future.
The new toolbox
Optogenetics
A recent article published by the New Yorker profiles Karl Deisseroth, a psychiatrist and neuroscientist at Stanford University. In the article Deisseroth mentions the difficulty in treating and understanding neurologic and psychiatric disorders, asking: "When we have the complexity of any biological system—but particularly the brain—where do you start?".
Dr.Deisseroth is known for more than just treating patients. He is one of the inventors of one of the most exciting and cutting-edge experimental techniques used in neuroscience today – optogenetics. Optogenetics is a technique that allows expression of light-sensitive channels in neurons. By combining this technique with genetic and viral approaches, researchers can insert these channels into very specific populations of neurons. Ultimately, this approach allows researchers to control distinct groups of neurons and individual circuits of the brain by using flashes of light, providing unprecedented control on cellular and circuit function.
The study of neural circuits underlying behaviors has been a main aim of the field of neurobiology. Various circuits that underlie many human behaviors and cognitive functions are now known. Also, the specific circuits that are affected in psychiatric illnesses are starting to be uncovered. Applying optogenetics to the study of these disorders will provide researchers with a much more accurate approach to probing how various circuits function in models of neuropsychiatric disorders without affecting surrounding circuits. This is important, as non-specific circuit stimulation can actually cause confounding results.
iPSC
The advent of induced pluripotent stem cells (iPSC), a method published by Shinya Yamanaka's group in 2007 in which skin cells can be reverted to a stem cell-like state via the expression of "reprogramming factors", now provides a means of allowing researchers to use human cells to study disease. iPSC's can be driven to form various cell types, including neurons, by exposing these cells to a cocktail of factors known to be important in driving the development of nervous tissue.
Before the discovery of iPSCs by the Yamanaka group, the only available way of studying human brain tissue was through the use of post-mortem tissue and human embryonic stem cells. Now, iPSC technology allows researchers to collect skin cells from large groups of patients via skin biopsy, samples that can then be used to form patient-specific neurons. These neurons can be derived from actual patients with mental disorders, allowing researchers to study these diseases using human neurons from these patients.
Cerebral Organoids
Understanding the neural circuits that are disrupted in neuropsychiatric disorders remains a huge goal for neuroscience research. This is highlighted by the BRAIN initiative, put forth by the Obama administration in 2013, an initiative that aims at understanding how individual cells and neural circuits work together in order for researchers and clinicians to better understand brain disorders. A few years ago, approaches to studying and probing brain circuits, even by optogenetics, was limited to animal models, because cells grown in a culturing dish in a lab fail to form the neural circuits that are observed in the brain. But a paper published in 2013 from the Knoblich lab, showed that iPSC-derived neurons can be used to create "cerebral organoids", small bits (4mm diameter) of neural tissue that were found to express markers characteristic of various brain regions including cortical and hippocampal regions. Since the publication of this innovative technique, other groups have published similar methods, creating additional versions of 3-D neural cultures and even making cerebellar-like structures, a brain structure known to be important for movement and coordination. In fact, WIRED magazine recently published an article discussing a recent paper that created what the authors call "cortical spheroids" (and what WIRED calls "brain balls"), a different method for developing organoid-like structures. This technique cannot yet be used to study neural circuits as they truly exist in the actual mouse or human brain (the circuits and brain-like regions observed in culture are very rudimentary). However, the advancement of these techniques over the next decade or two could provide new and exciting ways to probe actual human brain circuits using patient cells.
Though every day we gain greater insight into how the human brain works and how brains might be disrupted in psychiatric disorders, we are far away from uncovering the exact circuits and mechanisms that underlie each of these disorders. It is clear that tools such as optogenetics, iPSC-derived neurons, and cerebral organoids can be used to provide tremendous control and detailed study of human neurons from these patients. Together, these studies might be able to gain a better understanding of how human neurons and neural circuits go awry in these disorders; leading to identification of novel targets for the development of drug therapies, providing promise for these patients and finally allowing scientists and clinicians to uncover the biology behind mental illness.
More information: References
1. Uhlhaas, P. J. & Singer, W. Abnormal neural oscillations and synchrony in schizophrenia. 1–14 (2010).
2. Brandon, N. J. & Sawa, A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. 1–16 (2011).
3. Green, E. K. et al. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Molecular Psychiatry 15, 1016–1022 (2009).
4. Mei, L. & Xiong, W.-C. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nature Publishing Group 9, 437–452 (2008).
5. Gauthier, J. et al. De novo mutations in the gene encoding the synaptic scaffolding protein SHANK3in patients ascertained for schizophrenia. Proceedings of the National Academy of Sciences 107, 7863–7868 (2010).
6. Feng, Y. & Walsh, C. A. Protein-protein interactions, cytoskeletal regulation and neuronal migration. Nat Rev Neurosci 2, 408–416 (2001).
7. Lewis, D. A., Curley, A. A., Glausier, J. R. & Volk, D. W. Cortical parvalbumin interneurons andcognitive dysfunction in schizophrenia. Trends in Neurosciences 35, 57–67 (2012).
8. Takahashi, K. et al. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 131, 861–872 (2007).
9. Dolmetsch, R. & Geschwind, D. H. The Human Brain in a Dish: The Promise of iPSC-Derived Neurons. Cell 145, 831–834 (2011).
10. Lancaster, M. A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013).
11. Paşca, A. M. et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nature Chemical Biology (2015).
Provided by Public Library of Science