Deep sea light shines on drug delivery potential
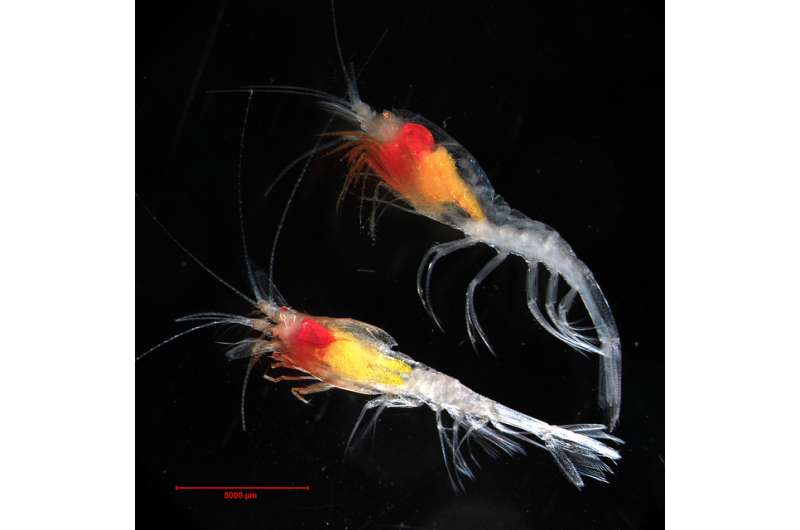
A naturally occurring bioluminescent protein found in deep sea shrimp—which helps the crustacean spit a glowing cloud at predators—has been touted as a game-changer in terms of monitoring the way drugs interact with live cells.
Harry Perkins Institute of Medical Research Associate Professor Kevin Pfleger and his colleagues have successfully tested NanoLuc's interactions in live cell cultures and published their work in this month's issue of Nature Methods.
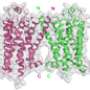
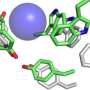
They used bioluminescence resonance energy transfer (BRET) with NanoLuc fused to G protein-coupled receptors (GPCRs) to monitor how ligands like natural hormones or drugs bind to these cell surface receptors.
"GPCRs are the target of at least 30 per cent of drugs on the market," he says.
Nanoluc represents a powerful fraction of the native bioluminescent luciferase enzyme, which forms part of the shrimp's defence system.
In the new method, known as NanoBRET, NanoLuc is coupled to a target receptor which can trigger energy transfer when a fluorescently tagged ligand is bound to the NanoLuc-receptor complex.
"This then results in light being emitted, like turning on a molecular light bulb," he says
"You are able to look at a bright specific signal that comes from a [biological] interaction in real time while normal cell functioning is happening."
Dr Pfleger says the luciferase normally used for this type of experiment simply did not work.
Unlike NanoLuc, it could not properly position itself outside of the cell membrane to function correctly.
BRET method could aid further drug discoveries
He says the principle of BRET works just as well for other receptor–ligand interactions and drug discovery researchers working on other drug targets could also benefit from using NanoLuc.
"Understanding how ligands bind to receptors, as well as the strength and potency of binding, is incredibly important for drug discovery and development," Dr Pfleger says.
"These binding characteristics are indicative of how much drug would be needed to give an optimal treatment effect."
Dr Pfleger says allosterism, the concept that drugs do not necessarily have to bind to the exact same site as where the natural hormone binds, is the future in pharmacology.
"If cell function is like playing the piano, most drugs slam the keys or close the lid," he says.
"Allosteric modulation provides possibilities to get back the tune the body is trying to play."
Dr Pfleger will use NanoBRET to further investigate the nuances around how and where different drugs bind to receptors and how this can have a fine-tuning influence on cell function.
More information: "Application of BRET to monitor ligand binding to GPCRs." Nature Methods 12, 661–663 (2015) DOI: 10.1038/nmeth.3398
"Engineered Luciferase Reporter from a Deep Sea Shrimp Utilizing a Novel Imidazopyrazinone Substrate." ACS Chem Biol. 2012 Nov 16; 7(11): 1848–1857. Published online 2012 Aug 15. doi: 10.1021/cb3002478