September 10, 2015 report
Closer look at proteins involved in Parkinson's disease reveals segment involved in amyloid formation
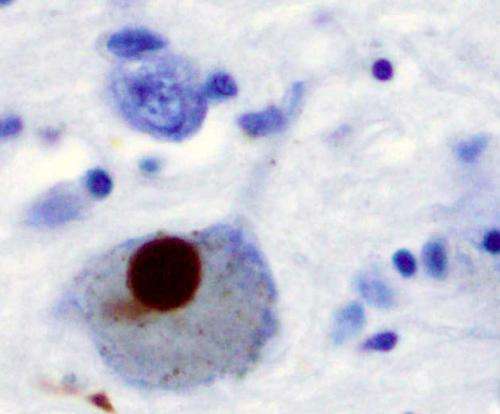
(Medical Xpress)—A team of scientists from several research centers in the U.S. has taken a closer look at α-synuclein, a protein that is abundant in the human brain, and which is also involved in the development of Parkinson's disease. In their paper published in the journal Nature, the team describes their study and offers some new ideas on how neurodegenerative diseases may come about due to the formation of fibrils. Michel Goedert of Cambridge University in the U.K. and Yifan Cheng with the Howard Hughes Medical Institute offer a News & Views piece on the work done by the team and how it has added to a better understanding of how diseases such as Parkinson's get their start.
α-synuclein is a protein found most often in the tips of nerve cells and is believed to be involved in communication between neurons via lipid binding. In some cases, however, α-synuclein performs abnormally and causes fibrils to form—such fibrils tend to migrate causing damage to brain tissue and associated neurodegeneration—this is what happens in Parkinson's disease, which is still incurable. In this new study, the researchers took a closer look at a stretch of amino acids that are part of the makeup of α-synuclein—peptides corresponding to residues 68–78. Noting that crystals that form the core of them are too small to view with optical microscopy (because they are smaller than the wavelength of light) the team instead used micro-electron diffraction. Doing so allowed them to see not just the peptides, but pairs of face-to-face β-sheets, which are believed to be the building blocks of fibrils.
Most prior research has implicated residues located between 30 and 53 as the main culprit involved in the development of Parkinson's disease, but now, according to the results of this new research, it appears that residues between 68 and 78 may play a role as well. The researchers suggest that this region may interact with the more studied region in ways that cause enhanced fibril formation to occur.
Goedert and Cheng note that the newly uncovered structural information could help in the development of molecules able to inhibit the formation of α-synuclein fibrils, and thus serve as a means of stopping the progression of such degenerative diseases.
More information: Structure of the toxic core of α-synuclein from invisible crystals, Nature (2015) DOI: 10.1038/nature15368
Abstract
The protein α-synuclein is the main component of Lewy bodies, the neuron-associated aggregates seen in Parkinson disease and other neurodegenerative pathologies. An 11-residue segment, which we term NACore, appears to be responsible for amyloid formation and cytotoxicity of human α-synuclein. Here we describe crystals of NACore that have dimensions smaller than the wavelength of visible light and thus are invisible by optical microscopy. As the crystals are thousands of times too small for structure determination by synchrotron X-ray diffraction, we use micro-electron diffraction to determine the structure at atomic resolution. The 1.4 Å resolution structure demonstrates that this method can determine previously unknown protein structures and here yields, to our knowledge, the highest resolution achieved by any cryo-electron microscopy method to date. The structure exhibits protofibrils built of pairs of face-to-face β-sheets. X-ray fibre diffraction patterns show the similarity of NACore to toxic fibrils of full-length α-synuclein. The NACore structure, together with that of a second segment, inspires a model for most of the ordered portion of the toxic, full-length α-synuclein fibril, presenting opportunities for the design of inhibitors of α-synuclein fibrils.
© 2015 Medical Xpress