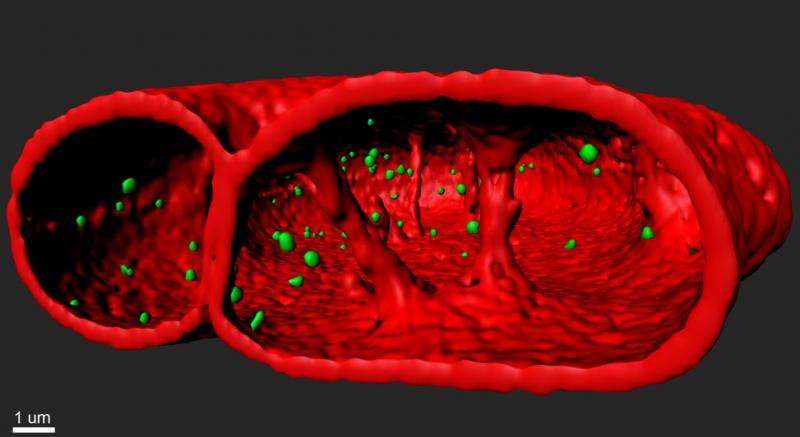
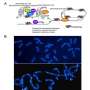
3D-rendering of the nuclear lamina (red) and telomeres(green) in human cells expressing the mutated form of lamin A, causing the accelerated ageing syndrome progeria. Credit: A*STAR
Scientists from A*STAR's Institute of Medical Biology (IMB) have successfully established a comprehensive model of rare accelerated ageing syndrome Hutchinson-Gilford Progeria Syndrome (HGPS), thereby opening up the possibility of curing HGPS, with far-reaching implications on ageing and human health. The ground-breaking study was published in eLife in September 2015.
HGPS is an extremely rare genetic disease which causes patients to start ageing rapidly when they are around a year old. Symptoms include stunted growth and joint abnormalities, and patients often die of heart failure by their teenage years. One in four million children suffer from HGPS, which currently has no effective treatment. The syndrome is caused by a mutated protein called progerin, which induces DNA damage, triggers premature cellular ageing and slows down cell proliferation, resulting in accelerated ageing. It was previously thought that progerin did so through causing the nucleus to be deformed, thereby weakening the ability of cells to divide and proliferate. But there is more to HGPS than meets the eye.
This study proposes a radically different model, in which progerin is linked to telomeres – repetitive DNA sequences that protect the ends of human chromosomes and shorten each time a cell divides, thereby limiting cell regenerative capacity. Progerin induces a reduction in heterochromatin, a tightly packed form of DNA, making telomeres in the cell more fragile and susceptible to damage. The damaged telomeres in turn trigger premature cellular ageing.
While a few studies had reported that telomerase appeared to rescue progerin-induced DNA damage, no study thus far had provided conclusive evidence or understood the underlying reasons. The study now demonstrates that telomeres are indeed closely related to HGPS and explains why. It was found that adding the enzyme telomerase, which elongates telomeres, comprehensively counteracted the effects of progerin through preventing proliferative defects, DNA damage, and gene expression differences induced by progerin. However, telomerase could not rescue the loss of heterochromatin. It was further found that, while increasing the protein LAP2alpha impaired the proliferation of normal cells, the same increase was instead able to pull up heterochromatin levels in progeric cells to match that of normal cells, thus preventing telomere damage in the first place. Taken together, the study reveals that LAP2alpha plays a key role in HGPS.
These findings may open new avenues for the clinical treatment of HGPS patients. Therapies incorporating LAP2alpha may be administered to early-diagnosis HGPS patients to minimise telomere damage, while older patients, who have already undergone telomere damage, may be provided with telomerase enhancing treatments to elongate their telomeres. Treating HGPS patients holistically with both LAP2alpha and telomerase would thus counteract the effects of progerin on cellular ageing, and greatly increase their chances of survival past their teenage years.
Furthermore, the study underscores the absolute criticality of telomeres to human health and ageing, given that telomere damage can cause accelerated ageing. Such findings pave the way towards the future possibility of slowing down the ageing process, through the development of telomerase enhancing drugs. At the same time, while telomere damage already occurs in normal ageing, increased damage has been associated with lifestyle choices such as smoking and obesity. Improving public awareness of lifestyle choices which may damage telomeres would thus contribute to managing the overall issue of ageing as well.
IMB Project Leader Dr Oliver Dreesen, who co-led the study with Professor Colin Stewart, stated, "When we embarked on this project, our goal was to identify novel biomarkers for ageing, and to understand the molecular mechanism why these kids grow old more quickly. I think this, and our previously published work, is a significant contribution to our understanding of human ageing. Furthermore, given the tremendous potential of telomeres to harm our health, we must be more careful in making choices that could possibly cause them damage."
Professor Birgit Lane, Executive Director of IMB, said, "It is very exciting that the team has made critical new inroads in the field of human ageing, as the ageing population is an increasingly pressing problem facing the world today. We hope that this new insight will contribute to improved solutions and management of ageing, and bring much benefit to society."
Professor Daniela Rhodes, Director of the Nanyang Technological University (NTU) Institute of Structural Biology and a global expert in the telomere and chromatin field, commented, "This excellent piece of work further highlights the importance of telomeres, DNA damage and cellular senescence in human health and disease. It also sheds light onto a novel mechanistic link between telomeres, chromatin structure and the nuclear lamina. I look forward to future exciting findings emerging from this research."