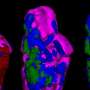
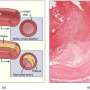
Researchers have developed and validated a new tool to help identify unstable or high risk atherosclerotic plaques—inflamed fatty deposits in the artery wall and a main contributor to cardiovascular disease (CVD). This breakthrough may lead to better identifying which plaques are considered at the highest risk for rupturing and causing a heart attack or stroke.
CVD remains the leading cause of morbidity and mortality in developed nations, despite advances in diagnosis and treatment. Atherosclerosis is an important contributor to CVD and varies in severity depending on multiple features that contribute to plaque progression and "stability."
Using an experimental model, researchers from Boston University School of Medicine (BUSM) and the University of California, San Diego have validated two novel, targeted fluorescent probes known as Activatable Cell Penetrating Peptides (ACPPs), for detecting the severity of atherosclerotic plaques. The findings appear in the journal PLOS ONE.
Atherosclerosis is a complex disease with many stages, ranging from plaques that can remain clinically silent for decades ("stable") to dangerous ("vulnerable") plaques. In their most highly advanced stage ("highest risk'), vulnerable plaques can suddenly disrupt to form a blood clot (thrombus) in the vessel, leading to myocardial infarction or stroke. Currently, various imaging methods are making advances in detecting vulnerable plaques although few focus on the important clinical endpoint of thrombosis.
"Our results showed that the fluorescence ACPP probes were able to distinguish high risk plaques with high sensitivity and specificity in vessels that have multiple plaques, many of which are stable, a common feature of human atherosclerosis that complicates the detection the most dangerous plaques", explained corresponding author James Hamilton, PhD, professor of physiology and biophysics and research professor of medicine at BUSM. "We are encouraged by our findings and plan to pursue the development of ACPPs with MRI probes for potential diagnostic applications in humans," he added.
Journal information: PLoS ONE
Provided by Boston University Medical Center