Celleron Therapeutics announces encouraging clinical results with new cancer drug CXD101
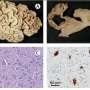
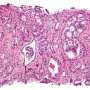
Celleron Therapeutics, the UK-based company developing personalised medicine for cancer patients, has today announced that significant clinical activity was observed in the first human trial of its pioneering personalised cancer treatment CXD101 in patients at Oxford's Churchill Hospital with advanced treatment-resistant aggressive disease. The results also indicate that CXD101 has favourable safety and tolerability.
'These results provide early clinical evidence that CXD101 is active against late stage cancer' commented Professor Nick La Thangue, Founder and Chief Scientist, Celleron Therapeutics and Professor of Cancer Biology in the Department of Oncology at Oxford University. 'CXD101 represents a new class of drugs with dual mode of action that not only targets tumour cells but also stimulates the patient's immune system to fight the cancer. These are extremely encouraging and important results and we look forward to driving the clinical trials forward as fast as possible in aggressive cancers using our personalised treatment approach'.
A major challenge in drug development is that all cancer patients respond differently to treatment. Clinical trials with Celleron's CXD101 drug are not only investigating the properties of the new drug but will also study a novel biomarker test, known as a companion diagnostic, to predict which patients can be successfully treated with the drug. This approach avoids the problem of treating patients who have little chance of benefiting from the treatment.
Dr John Whittaker, Celleron's Chief Operating Officer commented 'I am delighted to see Celleron, the Oxford Experimental Cancer Medicine Centre (ECMC) and Oxford Hospitals NHS Foundation Trust making excellent progress on Celleron's proprietary targeted therapeutic, CXD101, which opens up new and exciting opportunities for treating aggressive types of cancer'.
Dr Graham Collins, Haematology Consultant at the Churchill Hospital, Oxford, remarked ' These are very promising results with a very well tolerated drug demonstrating clinical activity in aggressive lymphomas '.
Professor Mark Middleton, Chief Investigator for the trial and Clinical Director at the University of Oxford, Department of Oncology noted 'Whilst there's a lot more work to be done, seeing patients benefit from CXD101 encourages us to study this exciting drug further. The support of the Experimental Cancer Medicine Centre has been key to developing and delivering the trial. It provides a way to bring new drugs to our patients, which might otherwise not happen'.
CXD101 is a next generation epigenetic immune-regulator representing a class of drug that kills cancer cells by blocking certain vital functions involved in gene expression, and reactivates the patient's immune system so that cancer cells can no longer evade immune recognition. The trial is a unique partnership between Celleron Therapeutics, Oxford University Hospitals NHS Foundation Trust and the Oxford Experimental Cancer Medicine Centre. The Oxford ECMC is led by Associate Professor Sarah Blagden.
More information: *The trial's entry on the UK Clinical Trial's Gateway can be found at Http://www.ukctg.nihr.ac.uk/trialdetails/NCT01977638