Researchers at Imperial College London have developed a tool that could change our understanding of one of the eye's critical systems. The results, which were part-funded by Fight for Sight, could make it easier to develop new drugs for glaucoma that target the main cause of elevated eye pressure.
High pressure in the eye – known as intraocular pressure – is the main risk factor for glaucoma. Sight loss from glaucoma is irreversible and reducing intraocular pressure is the only successful way to prevent further visual impairment.
Intraocular pressure is determined by a balance between the rate at which the eye makes and drains aqueous humour – the clear watery fluid that fills the front part of the eye. Elevated intraocular pressure is due to an unhealthy increase in the resistance to aqueous humour drainage from the eye.
In glaucoma, aqueous humour drainage becomes partly blocked and, like a clogged drain, pressure in the eye rises, analogous to an overflowing kitchen sink. Despite decades of research, no one has yet identified the clog that contributes to elevated pressure in the most common form of glaucoma.
Animal studies in mice are often used in research to study the mechanics that control drainage from the eye. However the small size of the mouse eye has made this technically challenging.
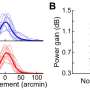
In this study, published in PLoS ONE, the research team describes iPerfusion for the first time. iPerfusion includes techniques for measurement, data analysis and presentation using a hydraulic device for pressure control, thermal flow sensor and automated computer interface. While it has been used here to study isolated eyes, the authors have already used the system in other species and with different biological tissues, such as lymph nodes. Using iPerfusion the team has shown a complex relationship between flow and pressure through the drainage pathway of mouse eyes. Whilst the measured drainage is zero when no pressure is applied, the flow rate increases sharply with increasing pressure. These results are unexpected and indicate that the drainage system responds to pressure, at least in healthy individuals. Ongoing work is characterising the pressure-flow relationship under conditions that mimic human glaucoma and in response to drugs that try to improve drainage.
Dr Darryl Overby of the Department of Bioengineering at Imperial College London led the study. He said:
"Our results show a clear non-linear relationship between flow and pressure in enucleated mouse eyes such that the commonly used linear model leads to a false impression that could have significant impact on the reliability of glaucoma research. "The study does have some limitations. Namely, the measurements were performed ex vivo, where the living eye may exhibit a different drainage pathway or an active response to pressure that becomes disrupted after isolation. While work is underway to address these limitations, we believe that iPerfusion is a transformative technology that will allow us to better identify drugs that target pore formation to increase outflow facility. Ultimately, better technologies lead to a better understanding of the eye, and upon this knowledge we can design and develop drugs that more successfully save vision in glaucoma patients."
Dr Dolores M Conroy, Director of Research at Fight for Sight, said: "The development of more effective treatments for glaucoma is the number one priority for patients and eye health professionals identified by the Sight Loss and Vision Priority Setting Partnership. iPerfusion will be key to the development of new treatments which target the outflow system – the root cause of increased intraocular pressure in glaucoma."
More information: Sherwood JM, Reina-Torres E, Bertrand JA, Rowe B, Overby DR. Measurement of Outflow Facility Using iPerfusion. PLoS ONE. 2016 Mar 7;11(3):e0150694
Journal information: PLoS ONE
Provided by Fight for Sight