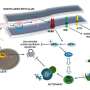
A major challenge in the field of neurodegeneration is the unclear understanding of neuronal dysfunction. Elucidation of these patho-mechanisms could result in the identification of novel therapeutic targets. In this article, Bell et al. present an exhaustive literature review highlighting the endoplasmic reticulum (ER) kinase PERK as a crucial contributor to systemic and neurodegenerative disorders. While the impact of PERK in various systemic diseases has been well characterized, its involvement in neurodegeneration is established to a lesser extent. PERK, and its downstream substrate eIF2?, are pathologically, genetically, and molecularly linked to several neurodegenerative disorders.
The active form of PERK, pPERK, is chronically upregulated in tauopathies such as Alzheimer's, Fronto-temporal dementia linked to chromosome 17, and Progressive Supranuclear Palsy (PSP). Genetically, a single nucleotide polymorphism in the gene coding for PERK, EIF2AK3, is associated with risk for PSP. Molecularly, pathogenic protein aggregates alter ER homeostasis triggering sustained PERK activation. Despite the reparative nature of its activity, long-term PERK activity activates pro-apoptotic cascades.
The objective of the review is to highlight the impact of PERK in neurodegenerative processes and underscore the pathway as a reservoir of therapeutic targets. To this end, current efforts attempting to inhibit PERK in in vivo models of tauopathy and prions have shown promising results. Therefore, PERK inhibition in these diseases is a promising therapeutic strategy, and efforts to develop optimal PERK inhibitors for the clinic are underway.
More information: Michelle Bell et al. PERK-opathies: An Endoplasmic Reticulum Stress Mechanism Underlying Neurodegeneration, Current Alzheimer Research (2016). DOI: 10.2174/1567205013666151218145431
Provided by Bentham Science Publishers