New sensitive method for early detection of amyloidosis in humans
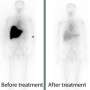
A team of scientists at Sweden's Linköping University has developed a molecular probe that can detect an array of different amyloid deposits in several human tissues. This new probe is extremely sensitive and was used at very low concentrations to correctly identify every positive amyloidosis sample when compared to the traditional clinical tests. The probe also picked up some amyloidosis signals that the traditional methods were unable to detect. This result means that the new probe could be used to detect amyloidosis before symptoms present, leading to faster and hence more effective treatment.
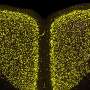
Amyloidosis is a general term for several different types of disease. Aggregates of amyloid proteins form and deposit in different tissues which can affect the normal function. As the disease progresses and amyloid deposits grow, tissues become irreversibly damaged. Amyloid deposits can be found in many different organs leading to a wide range of possible symptoms and making diagnosis challenging.
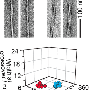
To date, the primary mode of diagnosis for amyloidosis has been the Congo red stain. However, evidence from the Linköping team, presented in Amyloid Journal show that their new probe is much more sensitive, being able to detect small amyloid deposits in samples that were previously determined to be amyloid-free.
"Given the sensitivity of the probe, we think this would make an excellent complement to traditional methods and could eventually be a replacement", says lead investigator Per Hammarström, professor at Linköping University.
According to the U.S. Office of Rare Diseases (ORD), a subsidiary of the National Institute of Health (NIH), amyloidosis is a rare disease, affecting less than 200,000 people in the U.S.. However, The Amyloidosis Foundation suspects that the figures are underreported and that amyloidosis is not that rare - just rarely diagnosed. A more sensitive diagnostic method would help to uncover the reality of the situation.
The Linköping team are optimistic about the use of the probe.
"It could also be used to identify new types of amyloids and presymptomatic patients who are at risk of developing the disease", says Hammarström and collaborator professor Peter Nilsson.
Research is continuing in this important field. In the future, the researchers hope to apply this to other diseases where amyloids are present and develop real-time, non-invasive diagnostic probes.
More information: Daniel Sjölander et al. Establishing the fluorescent amyloid ligand h-FTAA for studying human tissues with systemic and localized amyloid, Amyloid (2016). DOI: 10.3109/13506129.2016.1158159