FDA approves first pacemaker without wires from Medtronic
The Food and Drug Administration has approved the first pacemaker to do away with electrical wires that have long been a shortcoming of internal heart devices.
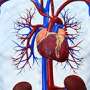
The one-inch Micra Transcatheter Pacing System works like older pacemakers to control irregular heartbeats, but does not use leads—wires that traditionally connect the device's generator to the heart. The wires can break, wear out or become infected and are the main weakness of such pacing systems.
Instead, the mini pacemaker from Medtronic PLC is implanted directly into the heart's lower-right chamber, which pumps blood to the lungs. The device is the size of a large pill and can be placed without surgery, through a tube into a blood vessel in the groin, and then up to the right side of the heart.
© 2016 The Associated Press. All rights reserved.