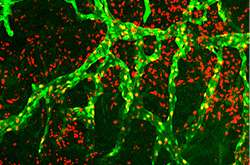
Lymphatic vessels overgrowing in mouse cornea in response to galectin-8. The lymphatic vessels are in green. Credit: Wei-Sheng Chen, Tufts University School of Medicine
After an injury to tissues, such as in organ transplantation, the body grows new lymphatic vessels in a process known as lymphangiogenesis. A new study in Nature Communications reveals a mechanism involved in the regulation of this process, specifically in corneal transplants and infectious eye disease. The team, led by researchers from Tufts University School of Medicine, the Sackler School of Graduate Biomedical Sciences at Tufts, and Tufts Medical Center, successfully prevented corneal inflammation, a condition that adversely affects transplantation, by inhibiting the overgrowth of these lymphatic vessels in a mouse animal model.
Lymphangiogenesis (pronounced "lymph" and then "angiogenesis) plays a significant role in organ transplant rejection, cancer metastasis, lymphatic obstruction (lymphedema), diabetes and hypertension. In recent years, because of the identification of lymphatic-specific markers, lymphangiogenesis has become a rapidly expanding field of research.
Lymphatic vessels are conduits for cells. In the eye, lymphatic vessels can overgrow in response to a corneal transplant. This triggers an influx of local "patrolling" cells from the cornea to the regional lymph nodes to initiate an immune response that can lead to corneal transplant rejection in high-risk patients. The new study focuses on the role of a protein, galectin-8, in the regulation of the growth of these lymphatic vessels.
The researchers from Tufts first determined that galectin-8 promotes the growth of new lymphatic vessels by a novel, carbohydrate-dependent mechanism and thus increases the risk of corneal transplant rejections. They then successfully identified approaches to inhibit galectin-8 and reduced the detrimental inflammatory lymphangiogenesis.
In separate tests, the research team also found that mice without the galectin-8 protein were more resistant to eye infections caused by herpes simplex virus. Previous studies had found that herpes simplex virus infections in the cornea (keratitis), a debilitating corneal infection, caused lymphangiogenesis. Importantly, the research team found that galectin-8 deficiency did not affect the overall health of the mice but played a key role in modulating the severity of inflammatory diseases.
The first author on the study is Wei-Sheng Chen, Ph.D., who did this work as part of his Ph.D. studies in Cell, Molecular & Developmental Biology at the Sackler School. He is now a postdoctoral fellow in senior author Noorjahan Panjwani's research lab at Tufts. Panjwani, Ph.D., is a professor in the department of ophthalmology at Tufts University School of Medicine and a member of three program faculties at the Sackler School.
"Galectin-8 is a potent lymphangiogenic factor and we hope that this knowledge contributes to new preventative treatments. We hope to explore it further to prevent corneal transplant rejection, but also to help find treatments for dry eye and other ocular diseases," said Panjwani. "These findings also potentially lay the foundation for a new approach to preventing a number of debilitating diseases, including non-eye organ transplant rejection, cancer metastasis, and lymphedema."
"High-risk corneal transplantation with very high rejection rates, and ocular surface tumors, such as conjunctival melanomas, have extremely limited medical therapies. The discovery of a novel anti-lymphangiogenic mechanism now paves the way for the development of novel therapeutic approaches for these devastating conditions," said co-author Pedram Hamrah, M.D., ophthalmologist at New England Eye Center, part of Tufts Medical Center.
"There is an unmet need for treatments to prevent lymphangiogenesis as well as to promote lymphangiogenesis. Preventing lymphangiogenesis could help prevent organ rejection and might contribute to reducing cancer metastasis. Promoting lymphangiogenesis could be helpful in preventing lymphedema, often a side effect of treatments for breast cancer," Panjwani continued. "There are numerous clinical trials for angiogenesis agents but few, if any, for lymphangiogenic agents."
"The molecular signaling involved in lymphangiogenesis is more complicated than previously thought. Studies on lymphangiogenesis have focused on the VEGF-C (growth factor)/VEGFR-3 (receptor for VEFG-C) pathway because it promotes both physiological and pathological lymphangiogenesis. We found that galectin-8 is sufficient to promote pathological lymphangiogenesis, surprisingly without the involvement of VEGFR-3. This means that galectin-8 might be an important target to prevent metastasis in addition to VEGF-C or VEGFR-3," said Chen.
"Although it requires future studies, it is possible that carbohydrate-based galectin-8 inhibitors could be used in the treatment of chronic inflammatory diseases, as well," he continued.
More information: Wei-Sheng Chen et al. Pathological lymphangiogenesis is modulated by galectin-8-dependent crosstalk between podoplanin and integrin-associated VEGFR-3, Nature Communications (2016). DOI: 10.1038/NCOMMS11302
Journal information: Nature Communications
Provided by Tufts University