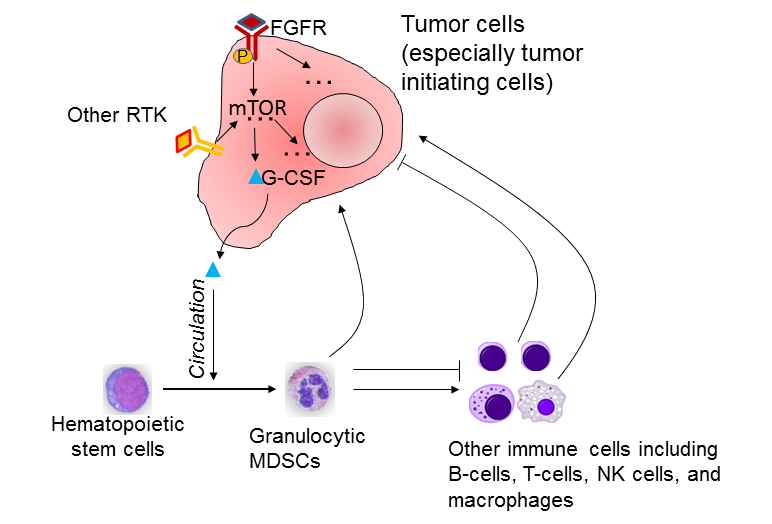
The mTOR pathway, an important pathway controlling cell growth and metabolism, is aberrantly activated in some breast tumors. This activation leads to increased expression of granulocytic colony formation factor (G-CSF, blue triangleEnglish ), which is then released into the circulation and stimulates the accumulation of myeloid-derived suppressor cells (MDSCs). MDSCs recruited to tumors directly enhance tumor initiation capacity. They also modulate the functions of other immune cells to dampen the anti-tumor immunity. RTK, receptor tyrosine kinase. Credit: X.Zhang
Not every breast cancer tumor follows the same path to grow. Some tumors have the assistance of myeloid-derived suppressor cells (MDSCs), a diverse type of immune cell involved in the suppression of the body's response against tumors. How breast cancer cells recruit MDSCs is not completely understood, but in a paper released today in Nature Cell Biology, Baylor College of Medicine researchers report a new mechanism that helps cancer cells engage MDSCs.
"There are alternative paths a tumor may take without the MDSCs, but those cancer cells that take the mTOR path of activity tend to have more MDSCs through the production of granulocyte-colony stimulating factor (G-CSF), which drives the accumulation of MDSCs," said corresponding author Dr. Xiang Zhang, a McNair Scholar and assistant professor of molecular and cellular biology at Baylor College of Medicine.
Knowing how cancer cells and MDSCs interact with each other helps researchers understand the events that may lead to tumor growth and metastasis and identify potential therapeutic targets. For instance, "determining that a patient's tumor is using the mTOR pathway would indicate that the cancer cells are more likely to depend on MDSCs for progression," said Zhang, who also is with the Lester and Sue Smith Breast Center at Baylor. "This information suggests that, in this case, available therapies for mTOR combined with therapies for MDSCs represent potential therapeutic strategies." Tumors that do not use the mTOR signaling pathway would not be expected to respond as well to the same therapies.
The discovery of Zhang and colleagues is much in line with the concept of personalized medicine. "People talk about the specific mutations one patient's tumor has that are not in another patient's tumor. The same type of tumors having different mutations may warrant different treatments; that is personalized medicine," explained Zhang. "We are trying to come from a different angle. We are trying to enrich this concept by saying that not only tumor-intrinsic characteristics are different from patient to patient, but, related to that, there is also diversity in terms of the immune components. Different tumors may evolve via different characteristics of the tumor and the immune response."
MDCSs are just one type of aberrant immune cell associated with the tumor. "In addition, there are other immune cells associated with the tumor - monocytes, macrophages, different subsets of T cells - that can either attack or help the tumor. All those cells may vary from patient to patient, and we don't really understand that yet," said Zhang.
In addition, MDSCs also play a role in non-cancer situations. For instance, in chronic inflammation, these cells try to suppress the inflammation; in this case, they play a pro-health role. So, "simply eliminating all MDSCs to treat cancer may likely result in negative side effects, such as autoimmune disease. That's why it's necessary to further characterize the diversity, to find the specific subsets of MDSCs that are tumor specific," said Zhang.
More information: Thomas Welte et al, Oncogenic mTOR signalling recruits myeloid-derived suppressor cells to promote tumour initiation, Nature Cell Biology (2016). DOI: 10.1038/ncb3355
Journal information: Nature Cell Biology
Provided by Baylor College of Medicine