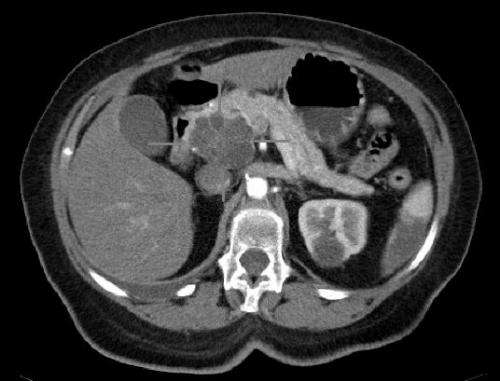
Axial CT image with i.v. contrast. Macrocystic adenocarcinoma of the pancreatic head. Credit: public domain
Massachusetts General Hospital (MGH) investigators have identified the first potential molecular treatment target for the most common form of pancreatic cancer, which kills more than 90 percent of patients. Along with finding that the tumor suppressor protein SIRT6 is inactive in around 30 percent of cases of pancreatic ductal adenocarcinoma (PDAC), the team identified the precise pathway by which SIRT6 suppresses PDAC development, a mechanism different from the way it suppresses colorectal cancer. The paper will appear in the June 2, 2016 issue of Cell and had been published online.
"With the advance of cancer genomics, it has become evident that alterations in epigenetic factors—those that control whether and when other genes are expressed—represent some of the most frequent alterations in cancer," says Raul Mostoslavsky, MD, PhD, of the MGH Cancer Center, senior author of the report. "Yet, not many of those factors have been described before, and those that have been identified have not been linked to specific downstream targets. Not only did more than a third of analyzed PDAC patient samples exhibit the molecular signature we identified, those patients also turned out to have very poor prognoses."
Among its other functions, SIRT6 is known to control how cells process glucose, and a 2012 study by Mostoslavsky's team found that its ability to suppress colorectal cancer involves control of a process called glycolysis. But while that study also found reduced SIRT6 expression in PDAC tumor cells, the current investigation indicated that SIRT6 deficiency promotes PDAC through a different mechanism. Experiments in cell lines and animal models revealed that low SIRT6 levels in PDAC were correlated with increased expression of Lin28b, an oncoprotein normally expressed during fetal development.
Lin28b expression proved to be essential to the growth and survival of SIRT6-deficient PDAC cells and acted by preventing a family of tumor-suppressing mRNAs called let-7 from blocking expression of three genes previously associated with increased aggressiveness and metastasis in pancreatic cancers. All of these hallmarks - reduced SIRT6, increased Lin28b and reduced let-7 expression - were found in tumor samples from patients who died more quickly.
"A general message from these studies is that cancer cells benefit from modulating epigenetic factors like SIRT6 by acquiring the ability to override normal cellular growth control patterns," says Mostoslavsky, an associate professor of Medicine at Harvard Medical School and an associate member at the Broad Institute. "Each tumor type may acquire a unique set of capabilities that may provide tumor-specific growth and survival advantages, which may need to be determined for each kind of cancer. In terms of our findings regarding PDAC, we are intrigued by the downstream pathways controlled by Lin28b and how they increase aggressiveness and metastasis, and we are hopeful that developing in the future Lin28b inhibitors could benefit this subset of PDAC patients, who currently have very few treatment options."
More information: Cell, DOI: 10.1016/j.cell.2016.04.033
Journal information: Cell
Provided by Massachusetts General Hospital