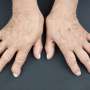
The results of a study presented today at the European League Against Rheumatism Annual Congress (EULAR 2016) showed that tiny particles made of a biodegradable polymer (BNPs—biodegradable polymer nanoparticles) have the potential to enable early detection and efficient long-term treatment of rheumatoid arthritis (RA), with minimal side effects. By coating these particles with a peptide that is only able to target inflamed joint tissue, BNPs may be used to efficiently and selectively deliver drugs and diagnostic probes into arthritic joints.
"Despite dramatic advances in the treatment of RA, currently available therapies can cause several side effects, many patients fail to respond, and true remission is only achieved in a minority," said Dr Paolo Macor lead investigator from the Department of Life Sciences, University of Trieste, Italy. "This is often due to late diagnosis of the pathology," he added.
"There is therefore a need to develop a new tool to enable early diagnosis, and also to develop tissue-specific agents able to reduce systemic side effects. This would increase the potency of the drug with lower doses, and also potentially reduce the cost of treatment," Dr Macor explained.
Unlike conventional drugs, BNPs can be designed to deliver therapeutic agents specifically to the site of inflammation. They also allow investigators to consider or reconsider therapeutic agents that were previously deemed too toxic to deliver through a systemic route. BNPs can also be filled with a contrast agent, such as gadolinium, and then used as a diagnostic tool for an early and functional diagnosis of joint inflammation. Early diagnosis and therapy has been identified as a potentially crucial step in achieving optimal control of disease progression and prognosis in RA.
Using an immunofluorescence technique, these peptide-coated BNPs were shown to preferentially target inflamed joint tissue. A single injection of targeted BNPs loaded with methotrexate completely resolved inflammation in a rat model of antigen-induced arthritis, while the same dose of free-methotrexate injected into the rat's bloodstream was ineffective.
Using a mouse model of chronic collagen-induced arthritis, an equivalent therapeutic effect was obtained comparing methotrexate-loaded BNPs and the same dose of free methotrexate with no toxic effects.
"The advantage of being able to deliver methotrexate in this targeted way is to be able to gain the benefits from this key treatment of RA, while reducing the risk of adverse effects that are more frequent at high doses," concluded Dr Macor.
Provided by European League Against Rheumatism