Good gut relationships key in defence against pathogens
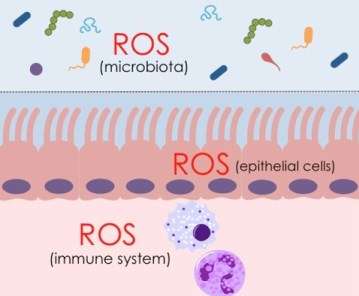
Fighting infection in the gut relies on a dynamic relationship between the cells that line gut walls and microbiota – the hundreds of different bacteria that co-exist within the human body without causing harm.
New research findings show how microbiota can adapt to compensate when early warning signalling by epithelial cells in the gut wall fails and threatens the first line of defence against pathogens.
Researchers in University College Dublin, the National Children's Research Centre and collaborators in University College Cork, Grenoble University Hospital Centre and Washington University School of Medicine published the findings of their study in the current issue of Cell Host & Microbe.
Reactive oxygen species (ROS) are chemically reactive molecules derived from oxygen. After pathogens are ingested by white blood cells, superoxide and related compounds are responsible for killing them. ROS are also released from the epithelial gut wall to disarm pathogens before they can even breach this barrier.
In instances where ROS levels drop, the defence system fails and the body is more susceptible to infection. This increases the risk of developing Crohn's-like disease as well as certain severe types of ulcerative colitis seen in very young children. It has also been shown to cause a severe immune disorder characterised by life-threatening infections.
UCD researchers led by Professor Ulla Knaus, UCD School of Medicine and Fellow of UCD Conway Institute set out to investigate the enzymes responsible for generating ROS in the gut to figure out how they contribute to the interactions triggered by infection.
When the team deleted these enzymes in the laboratory, they discovered that mice were still protected against infection due to changes occurring in the microbiota. Rather than using ROS to disarm bacteria, the defensive role is taken on by highly increased levels of peroxide-secreting strains of microbiota, with lactobacilli found to be particularly beneficial.
"The results of our study show that epithelial ROS are vital to shaping the composition of microbiota within our gut and, in turn, specific bacteria within this community can help to protect the body from gut pathogens", said Professor Knaus who led the study.
Lactobacilli are considered the model example of 'good' bacteria that have evolved with the human host and provide benefits to us such as anti-inflammatory effects. However, these mutually beneficial relationships between the human host and commensals can be disrupted by fat- and sugar-rich diets.
"If we can restore the species of bacteria to the gut that engage in stable mutualistic relationships, we may be able to protect the body from intestinal disease."
More information: Gratiela Pircalabioru et al. Defensive Mutualism Rescues NADPH Oxidase Inactivation in Gut Infection, Cell Host & Microbe (2016). DOI: 10.1016/j.chom.2016.04.007