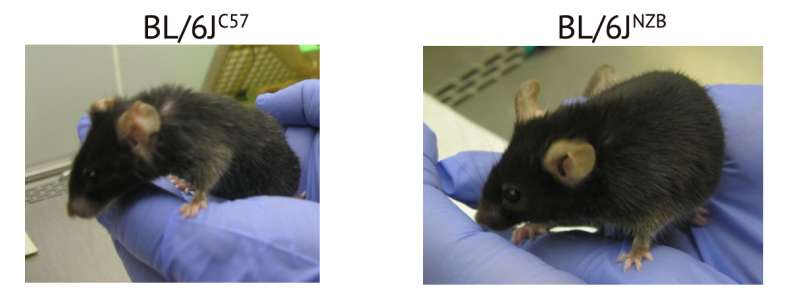
Mice have a life expectancy a little over 2 years. Both mice in the image are 2 years old and have been reared under the same conditions. Nonetheless the mouse on the right has aged normally and shows evident signs of superior health compared with its partner: it has more abundant and more lustrous fur, is more robust, has more muscular mass, and is more active. The only genetic difference between the mice is that they have each inherited a different healthy mitochondrial DNA variant from their mothers. These distinct variants of healthy (non-pathogenic) mitochondrial DNA have substantially different effects on the quality of aging. Credit: CNIC
The way we age might be determined long before the aging process starts and the first signs appear. Scientists at the Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), in partnership with groups at the universities of Zaragoza and Santiago de Compostela and the UK's Medical Research Council, have uncovered how the combination and interaction between our two genomes, the nuclear and the mitochondrial, triggers a cellular adaptation that has repercussions throughout our lives and determines how we age.
The study, led by Dr. José Antonio Enriquez, sheds light on the physiological differences between individuals and opens the way to the study of common aging-related conditions, such as diabetes, cardiovascular diseases, and cancer. The Nature study also provides extremely valuable information about how to best use mitochondrial donation technology. This therapeutic approach, popularly known for producing "three-parent babies", is designed to avoid the transmission of inherited pathogenic mutations and has already been approved in the UK.
Of the more than 20000 human genes, 37 are found not in the cell nucleus but in the mitochondria, small organelles that function as energy factories. The small mitochondrial genome, which we inherit from our mothers, is known as the mitochondrial DNA. Like its nuclear equivalent, the mitochondrial genome shows a degree of genetic variability, both in mice and humans.
The CNIC-led team found that non-pathogenic mitochondrial DNA variants have different impacts on organismal metabolism and aging. Dr. Enriquez explains that the study uncovers how "variation in just a few genes can determine whether we experience healthy aging." The results represent a major advance in our understanding of the aging process, showing that "non-pathogenic differences in mitochondrial function have direct repercussions on the pace of aging."
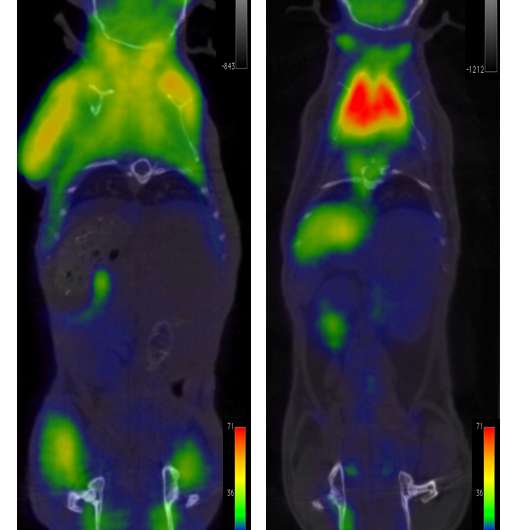
CT/PET scans of live mice, showing the stark differences in glucose uptake between the young mice differing only in the mitochondrial DNA variant inherited from the mother. Credit: CNIC
"The key to this study was understanding how the combination and interaction of our two genomes, the nuclear and the mitochondrial, triggers a cellular adaptation with repercussions throughout our lives," explains study first author Dr. Ana Latorre-Pellicer.
By using animal models, the research team obtained robust evidence that the simple measure of changing an animal's mitochondrial DNA triggers a series of adaptive cellular mechanisms in young animals that ensure a more healthy aging process. "If we can understand the biology underlying healthy aging that is free of age-associated diseases, we will be in a position to maintain long-lasting health during the aging process," affirms Dr. Latorre-Pellicer.
Three-parent babies
Mitochondrial donation technology has the potential to prevent the transmission of disease-causing mitochondrial DNA. This therapeutic approach, aimed at avoiding transmission of heritable pathological mutations, consists of replacing the suboptimal maternal mitochondria with mitochondria from a healthy donor. However, the use of this technology, popular known for producing "three-parent babies," and which is already approved in the UK, requires a thorough understanding of the physiological impact of mitochondrial DNA variability.
The results of the study underline the importance of making sure that the donor mitochondrial DNA in mitochondrial donation procedures is an appropriate match for the recipient's nuclear genome. The CNIC researchers are keen to stress that the potential risks of this procedure should not be ignored. "Just as with organ transplantation and blood transfusion, it is important to select mitochondrial donors, to ensure that the new mitochondrial DNA is genetically similar to that of the mother whose eggs require mitochondrial DNA replacement," concludes Dr. Enriquez.
More information: Ana Latorre-Pellicer et al, Mitochondrial and nuclear DNA matching shapes metabolism and healthy ageing, Nature (2016). DOI: 10.1038/nature18618
Journal information: Nature
Provided by Centro Nacional de Investigaciones Cardiovasculares