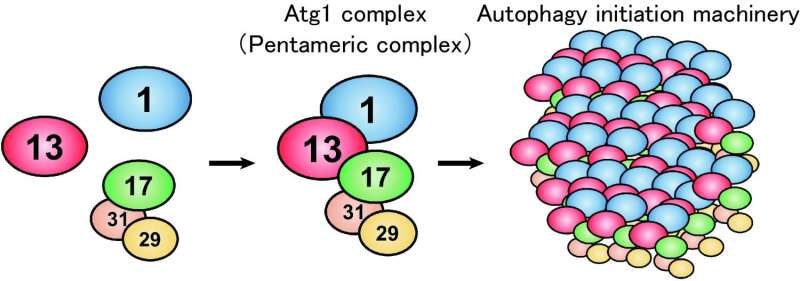
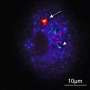
Under nutrient-rich conditions, Atg1, Atg13 and the Atg17-29-31 subcomplex exist without interacting with each other. Upon autophagy induction, under conditions such as starvation, they form a pentameric complex, which further assembles into a giant autophagy initiation machinery complex. Credit: Developmental Cell
In order to live, it is necessary for creatures not only to synthesize essential components, but also degrade harmful or superfluous components. Autophagy, an intracellular degradation system conserved among eukaryotes from yeast to humans, contributes to cell homeostasis via isolating and degrading various unnecessary components within cells. Since autophagy dysfunction is linked to severe diseases such as neurodegeneration and cancer, the artificial control of autophagy promises to facilitate the development of therapeutic and preventive treatment for these and severe diseases.
In budding yeast, the Atg1 complex, comprising Atg1, Atg13, Atg17, Atg29 and Atg31, mediates the initiation of autophagy (Figure 1). When autophagy is induced by starvation, these five components gather to form a dimer of pentamers. However, the formation of the dimer of pentamers alone is not sufficient for autophagy induction: and dozens of the pentameric complexes need to assemble into a supramolecular complex of autophagy initiation machinery elements. However, the molecular mechanism has been elusive.
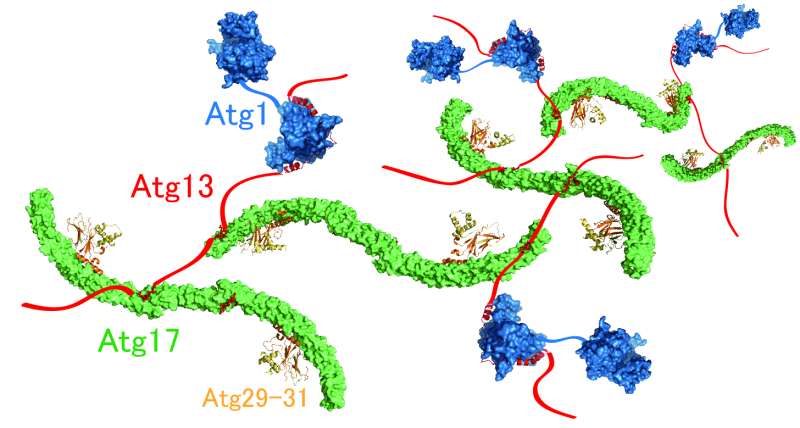
This mystery has been addressed by Honorary Prof. Yoshinori Ohsumi and Dr. Hayashi Yamamoto at Tokyo Institute of Technology, and Drs. Nobuo N. Noda and Yuko Fujioka at Institute of Microbial Chemistry, in collaboration with other researchers. The researchers focused on Atg13 and analysed its structure and function in vitro. The data revealed that Atg13 has an intrinsically disordered, string-like conformation in solution and that Atg13 has two distinct binding regions for Atg17. Detailed analyses of the interaction between Atg13 and Atg17 by X-ray crystallography uncovered that Atg13 links two Atg17 molecules to each other using two binding regions. Analysis of the size of the Atg1 complex revealed that Atg1 complexes are linked to each other by Atg13, which results in the formation of a huge autophagy initiation complex (Figure 2). When point mutations that impair the formation of this giant complex were introduced to Atg13, association of the autophagy initiation machinery was completely blocked. These data suggest that the supramolecular complex resulting from the linkage of Atg1 complexes to each other by Atg13 functions as the autophagy initiation machinery in vivo.
Atg13 (red) links Atg1 (blue) and two Atg17s (green) to each other, thereby promoting formation of the giant complex. Credit: Developmental Cell
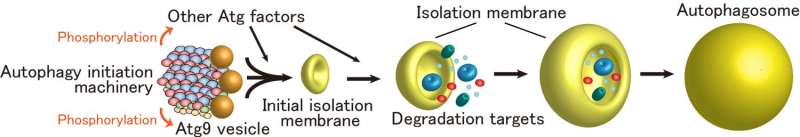
Next, the function of the autophagy initiation machinery was studied in budding yeast, revealing that the phosphorylation activity of Atg1 is markedly enhanced when it is incorporated into the supramolecular autophagy initiation complex. Moreover, it was revealed that the autophagy initiation machinery efficiently recruits Atg9 vesicles, which serve as membrane sources for autophagosome formation, and phosphorylates Atg9. These data collectively suggest that the autophagy initiation machinery not only mediates formation of initial isolation membrane through the recruitment of Atg9 vesicles, but also promotes autophagosome formation via phosphorylating autophagy-related factors including Atg9 (Figure 3).
These results are an important step in our quest to fully understand the molecular mechanisms of autophagy initiation and autophagosome formation. A better understanding of the mechanisms of the initial steps of autophagy promises to allow the development of compounds that specifically regulate autophagy, with exciting clinical implications for the treatment of a range of serious conditions.
The autophagy initiation machinery mediates formation of the initial isolation membrane through the recruitment of Atg9 vesicles. At the same time, the machinery phosphorylates Atg factors including Atg9, thereby promoting autophagosome formation. Credit: Developmental Cell
More information: DOI: 10.1016/j.devcel.2016.06.015
Provided by Tokyo Institute of Technology