Researchers discover how human immune receptors become activated in the presence of harmful substances
In George Orwell's classic dystopian novel Animal Farm, as the barnyard devolves into chaos the slogan "all animals are equal" quickly becomes "all animals are equal but some animals are more equal than others".
The same might be true for the tiny immune receptors scattered across the surface of our T-cells. Before now, it was unclear how these complex molecular receptors recognised harmful invaders (or antigens) and sent warning signals into the cell. It was largely assumed that "all receptors were equal".
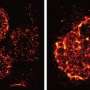
But a "Eureka moment" inside the UNSW Single Molecule Science lab has flipped this assumption. Using powerful imaging technology and some of Australia's only super-resolution microscopes that can zoom in to the level of a single molecule, researchers have viewed this critical first-stage in the immune response, inside a single functional T-cell, in unprecedented detail.
"Our findings have a touch of Animal Farm," says UNSW Scientia Professor Katharina Gaus, who oversaw the research. "Although all receptors in a single T-cell are genetically and biochemically identical, they are not functionally identical."
Despite being bombarded with antigens, the UNSW team found that only 25 percent of receptors on the T-cell were activated at a given time. Importantly, they found that this performance disparity was linked to spatial organisation on the cell's surface.
"If they're clustered together in a crowded environment they're much more likely to switch on than a receptor with no neighbours around it," says Dr Sophie Pageon, the study's lead author.
The team's findings, published today in the Proceedings of the National Academy of Sciences, describe a novel analysis method to distinguish signalling from non-signalling receptors in the same T-cell. This provides a valuable pathway to turn vital receptors back on and improve our immune response to deadly infections and cancers.
"Without reprogramming or genetically changing the whole T-cell, we can tune its sensitivity by corralling the receptors together, so they are densely clustered on the surface of the cell in a more optimal distribution," says Professor Gaus.
"In people with cancer, for example, T-cells eventually become inactive or exhausted. Taking what we now know about the T-cell clusters, we can develop strategies to rescue these T-cells, and turn the receptors back on."
She says her team has already developed a nanotechnology device that can re-arrange receptors on T-cells. Pending funding outcomes, they will begin experiments in mouse models, and should have a proof-of-principle ready within three years.
The crucial first stage of the immune response
The hallmark of an adaptive immune system is the ability of T-cells to recognise antigens, or foreign substances, that are potentially harmful. Tiny receptors on the external surface of T-cells bind to the antigens, and translate biochemical activity outside the cell into warning signals, which are passed intracellularly to the nucleus. The nucleus then activates the program's response and the killing of the infected cell or cancer cell.
"But these receptors do more than just flick a switch, to tell the cell 'yes or no'," says Professor Gaus. "It's almost like they have an artificial intelligence. They translate the complex biochemical binding event outside the cell into a warning signal, and they encode the level of response that's needed to effectively counteract the threat at the exact right time."
This is vital: should the immune system overreact, the body's T-cells might actually begin to attack our tissues and make us sick. On the other hand, if the immune system underreacts, we become more vulnerable to infections.
"It's quite astonishing. The quality control of the whole immune response happens at this molecular level," she says. "What sets our lab apart is that we are able to pinpoint this process by imaging individual molecules in single T-cells, and going right down to the molecular level to see how this mechanism works."
More information: Functional role of T-cell receptor nanoclusters in signal initiation and antigen discrimination, PNAS, www.pnas.org/cgi/doi/10.1073/pnas.1607436113