Some of the A*STAR team involved in the project: (left to right) HuiYin Lee, Anna-Marie Fairhurst, Susannah Thornhill, Teja Celhar from SIgN; and Richard Hopkins and Leigh Jones from the Connolly lab at IMCB. Credit: A*STAR Institute of Molecular and Cell Biology and A*STAR Singapore Immunology Network
The crucial role of dendritic cells in a fatal renal condition of systemic lupus erythematosus (SLE) has been exposed by A*STAR researchers. "Our studies show that these cells switch mild autoimmune phenotypes to severe kidney disease," says Anna-Marie Fairhurst at the A*STAR Singapore Immunology Network, who led the study.
SLE is a complex autoimmune disorder that predominantly affects women of child-bearing age. The disease is characterized by a range of gradually worsening symptoms, including joint pain, heart inflammation, and a butterfly-shaped rash across the nose and cheeks. A third of patients will develop life-threatening kidney disease, which Fairhurst has been trying to trace back to its pathological origins.
The immune cells of healthy individuals express a protein, called Toll-like receptor 7 (TLR7), that helps them recognize foreign pathogens. When hyper expressed in mouse models of SLE, however, TLR7 directs kidneys to their death. Fairhurst wondered which specific immune cells helped precipitate this decline. In an earlier study, she had eliminated the role of B cells, so her next suspect was the antigen-presenting dendritic cells.
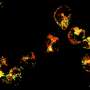
Fairhurst and her colleagues doubled TLR7 expression levels in a mouse model of SLE and then selectively eliminated the surplus receptors from individual cell populations. When they deleted TLR7 in B cells, the disease continued to progress. But, this time, when they deleted TLR7 in dendritic cells, the disease stopped and they observed no inflammation in the kidneys (see image).
Further analysis revealed that a specific type of dendritic cell, known as conventional CD11b+, was primarily responsible for infiltrating the kidneys and causing the disease.
The problem with these findings was that the same dendritic cells in humans typically do not express TLR7. This raised a troubling question for Fairhurst about their role in human disease.
To test their relevance, she isolated dendritic cells from blood samples of healthy individuals and prodded them into expressing TLR7 using heat inactivated or live flu viruses, and a protein known to stimulate an immune response called interferon-alpha. Surprisingly, live influenza and interferon-alpha increased TLR7 expression in the dendritic cells.
Fairhurst plans to analyse blood samples from human patients of SLE to chart TLR7 expression levels for different manifestations of autoimmunity. Doctors recommend annual flu vaccines for SLE patients, but Fairhurst wants to investigate different vaccination strategies to determine which are the most beneficial.
"More than two-thirds of SLE patients 'in remission' still suffer and take daily medication," says Fairhurst. "We hope to make some changes in this process."
More information: Teja Celhar et al. RNA sensing by conventional dendritic cells is central to the development of lupus nephritis, Proceedings of the National Academy of Sciences (2015). DOI: 10.1073/pnas.1507052112
S.-H. Hwang et al. B Cell TLR7 Expression Drives Anti-RNA Autoantibody Production and Exacerbates Disease in Systemic Lupus Erythematosus-Prone Mice, The Journal of Immunology (2012). DOI: 10.4049/jimmunol.1202195
Journal information: Proceedings of the National Academy of Sciences , Journal of Immunology