Model maps out molecular roots of learning and memory formation
A team of researchers has built a mathematical model that describes the molecular events associated with the beginning stage of learning and memory formation in the human brain.
The research, published in the journal Proceedings of the National Academy of Sciences, paves the way for understanding cognitive function and neurodegenerative diseases—at the molecular and cellular levels.
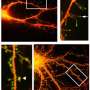
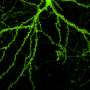
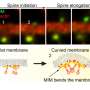
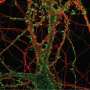
The study focuses on the dynamics of dendritic spines, which are thorny structures that allow neurons to communicate with each other. When a spine receives a signal from another neuron, it responds by rapidly expanding in volume—an event called transient spine expansion.
Transient spine expansion is one of the early events leading up to learning and memory formation. It consists of a cascade of molecular processes spanning four to five minutes, beginning when a neuron sends a signal to another neuron.
Many of the molecular processes leading up to transient spine expansion have already been identified experimentally and reported in the literature. Here, the authors built a map of many of these known processes into a computational framework.
"Spines are dynamic structures, changing in size, shape and number during development and aging. Spine dynamics have been implicated in memory, learning and various neurodegenerative and neurodevelopmental disorders, including Alzheimer's, Parkinson's and autism. Understanding how the different molecules can affect spine dynamics can eventually help us demystify some of these processes in the brain," said Padmini Rangamani, a mechanical engineering professor at the University of California San Diego and first author of the study.
"This work shows that dendritic spines, which are sub-micrometer compartments within individual neurons, are the prime candidates for the initial tag of transient, millisecond synaptic activity that eventually orchestrates memory traces in the brain lasting tens of years," said Shahid Khan, senior scientist at the Molecular Biology Consortium at Lawrence Berkeley National Laboratory and a co-author on the PNAS paper.
In this study, researchers constructed a mathematical model, based on ordinary differential equations, linking the different molecular processes associated with spine expansion together. They identified the key components (molecules and enzymes) and chemical reactions that regulate spine expansion.
As a result, they observed an interesting pattern—that the same components could both turn on and off some of the steps in the sequence—a phenomenon called paradoxical signaling. Further, they linked the chemical reactions of the different molecules to the reorganization of the actin cytoskeleton, which gives the cell its shape.
Both of these features—paradoxical signaling and linking spine expansion to actin reorganization—make this model robust, Rangamani explained. "By putting all these complicated pieces together in a simple mathematical framework, we can start to understand the underlying mechanisms of spine expansion. This is one of the benefits of combining mechanics of the cytoskeleton and biochemistry. We can bring together pieces of experimental work that are often not seen. However, we should note that we are only at the beginning stages of understanding what spines, neurons and the brain can do."
"This work is notable for bringing together aspects from diverse disciplines (systems biology, cell signaling, actin mechanobiology and proteomics) and should motivate similar multi-disciplinary efforts for other problems in fundamental cellular neuroscience," Khan said.
Rangamani started this research as a postdoctoral fellow in the lab of George Oster, professor emeritus of cell and developmental biology at the University of California, Berkeley and senior author of the study. She continued this work and incorporated it into her research program at the Jacobs School of Engineering at UC San Diego.
More information: Padmini Rangamani et al, Paradoxical signaling regulates structural plasticity in dendritic spines, Proceedings of the National Academy of Sciences (2016). DOI: 10.1073/pnas.1610391113