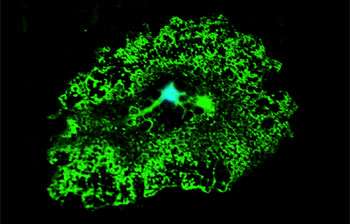
Oligodendrocytes are large cells whose main function is to produce myelin. Myelin wraps around the neurons and allows signals to run faster, thus facilitating the communication from one neuron to another. Here in green we managed to capture the wide myelin tree that develops from the central cell body. In MND these cells gain toxic properties and from friends they become enemies. Credit: University of Sheffield
A first-of-its-kind oligodendrocyte in vitro model shows that human cells normally supportive of motor neuron function play an active role in amyotrophic lateral sclerosis pathogenesis – and this discovery may point the way toward therapeutic timing and targets.
A number of studies over the last decade have shown that cells which normally support motor neurons, such as astrocytes and microglia, contribute to motor neuron death during the progression of amyotrophic lateral sclerosis (ALS). More recently, work with mouse models has shown that oligodendrocytes, also important to normal motor neuron function, are involved in ALS progression as well.
A new study from researchers at Nationwide Children's Hospital is the first to use oligodendrocytes derived from the skin of human ALS patients to demonstrate that these cells actually induce motor neuron death. The study, published in Proceedings of the National Academy of Sciences, also found that knockdown of the enzyme SOD1 in early ALS oligodendrocyte progenitors can lead to motor neuron rescue.
"We were able to dig deep in trying to make a bridge between a mouse model and what is happening in humans," says Brian Kaspar, PhD, senior author of the paper and a principal investigator in the Center for Gene Therapy at The Research Institute at Nationwide Children's. "We have been able to begin asking questions about how exactly oligodendrocytes go wrong and how they lead to motor neuron death."
The researchers developed a novel co-culture model that allowed observation of mouse and human ALS oligodendrocytes and their effect on motor neurons. In addition, the study partially relied on a "direct conversion" method pioneered in Dr. Kaspar's laboratory, which allows the programming of skin cells from living ALS patients to become neural progenitor cells.
A previous study by Dr. Kaspar's lab already showed that these skin-derived neuronal progenitor cells can efficiently be differentiated into astrocytes; the current study proves that the cells can become oligodendrocytes and presumably other cell types as well.
Oligodendrocytes were derived from human patients who had both familial ALS (that is, a family history of the disease) and the more common sporadic ALS (or no family history), and cells from both sources led to motor neuron death. Cells from healthy individuals or individuals suffering from other neuromuscular disorders did not harm motor neurons.
Mutations of the gene SOD1 have long been implicated in familial ALS, but studies from multiple laboratories including Dr. Kaspar's provided evidence that the gene may also have a role to play in sporadic ALS. The new study found that reducing levels of the SOD1 enzyme in progenitor cells before they become oligodendrocytes reduced the toxicity towards motor neurons leading to either greater survival or complete rescue. That was true in all familial and sporadic ALS samples with the exception of samples that carried C9orf72 gene mutations.
After the progenitors fully matured into oligodendrocytes, however, SOD1 knockdown did not result in motor neuron rescue.
"There is a point of no return," explains Dr. Kaspar, who is also a professor in the Department of Pediatrics and Department of Neuroscience at The Ohio State University College of Medicine. "When these cells have reached a certain point of differentiation or in their toxicity profile, you can't reverse it. This tells us to treat as early as possible, which is an emerging theme in many neurodegenerative disorders."
Direct conversion is an easier and faster method for deriving cells than the more traditional reprogramming to induced pluripotent stem cells (iPSCs). Moreover, there is increasing evidence that direct conversion allows the age-profile of a cell to be maintained, which is crucial when studying adult-onset neurodegenerative disorders, says Kathrin Meyer, PhD, a researcher in Dr. Kaspar's lab.
"The method is fast enough that we can derive and test these cells while a patient is still alive and eligible for clinical trials," Dr. Meyer says. "We now have even more proof that this method works, and we can use it to look at disease progression or at more specific differences between patient populations, thus developing more targeted disease treatments."
More information: K. Meyer et al. Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS, Proceedings of the National Academy of Sciences (2013). DOI: 10.1073/pnas.1314085111
Ferraiuolo L, Meyer K, Sherwood T, Vick J, Likhite S, Frakes A, Miranda CJ, Braun L, Heath P, Pineda R, Beattie C, Shaw PJ, Askwith C, McTigue D, Kaspar BK. Oligodendrocytes contributed to motor neuron death in ALS via SOD1 dependent mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2016 Sep 26. [Epub ahead of print]
Journal information: Proceedings of the National Academy of Sciences
Provided by Nationwide Children's Hospital