Research opens the possibility of new, noninvasive genetic prenatal testing
Researchers at Baylor College of Medicine, Drexel University College of Medicine, Texas Children's Hospital and RareCyte, Inc. have determined that it is feasible to develop a prenatal, noninvasive genetic test based on rare fetal cells that are present in the mother's blood. The study appears in Prenatal Diagnosis.
"These fetal cells were first reported in the circulation of pregnant women more than 40 years ago, and people have been hoping since that time to be able to use those cells for prenatal diagnosis," said senior author Dr. Arthur Beaudet, who is the Henry and Emma Meyer Chair of Molecular and Human Genetics and professor of molecular and cellular biology and of pediatrics at Baylor. "The technology changed over the years to an emphasis on developing methods to analyze the cells' DNA to get more genetic information about the fetus. With the emergence of RareCyte's platform technology for the identification of rare circulating cells and the retrieval of single cells, this is now possible."
Although other noninvasive methods for genetic testing are currently widely available, such as cell-free fetal DNA testing, they have their limitations. For instance, cell-free DNA testing cannot reliably detect very small changes in the fetal genome, in particular gene deletions that can lead to devastating diseases such as Angelman syndrome, a severe developmental disability. Beaudet anticipates that, if the test can become routine practice in current and future forms, it could be transformative for prenatal diagnosis, offering comparable information to that which can be obtained by amniocentesis and chorionic villus sampling.
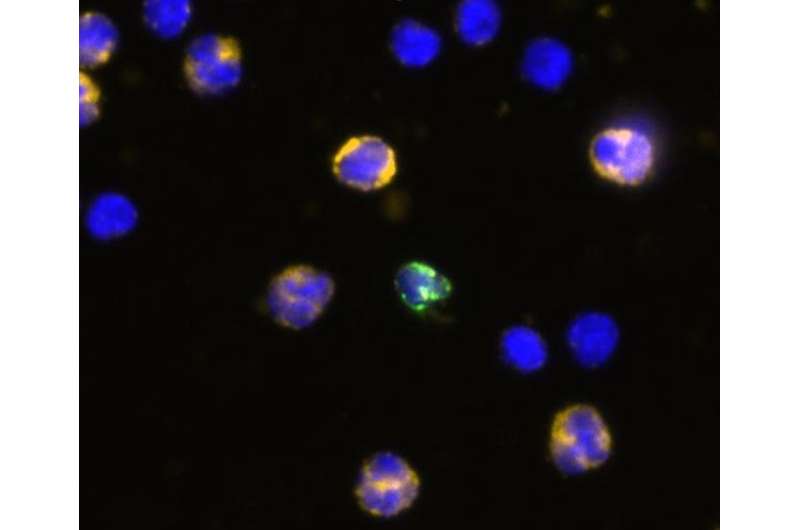
"Our goal in this project was to determine whether it was possible to collect trophoblasts present in the mother's circulation in numbers sufficient for further genetic analysis," said Beaudet. Trophoblasts are cells from the placenta and carry the fetus's entire genetic material.
The main obstacle to achieving this goal is that there are very few fetal cells in the mother's blood, and they are very fragile.
"About two tablespoons of blood (20 to 30 milliliters) has hundreds of billions of maternal red blood cells and hundreds of millions of white blood cells but only 20 to 40 fetal cells," said Beaudet. "We showed that we frequently can recover 3 to 10 or more fetal cells and analyze them in various ways, including next generation DNA sequencing. At present the group can only process 5 to 10 samples per week on a research basis, and they are focused on increasing this number so that the test could be offered more widely as a routine clinical test. There are still 5 percent of the tests where we cannot recover any cells, but a second blood sample might be collected in such cases."
In the fetal cells the researchers recover from a blood sample, they analyze a sampling of the whole genome of each of the cells separately. Their analyses can determine changes such as having three, instead of two, copies of chromosome 21, a genetic change that causes Down syndrome. They can also detect smaller changes such as the deletion of 5 to 6 million base pairs (DNA letters) that causes Angleman syndrome.
"We are looking only at gene changes that are known to result in devastating disability," said Beaudet.
At present the test only focuses on abnormalities that are 2 to 3 million base pairs in size, but future versions of the test should allow sequencing the entire exome or genome of multiple fetal cells.
"There are about 500 different genes that result in severe intellectual disability, if they undergo a new deleterious mutation," said Beaudet. "The severe impairments coming from mutations in these genes are overall 3 to 5 times more common than Down syndrome and much more disabling. It's not going to be about testing for IQ, hair color, high blood pressure or arteriosclerosis risk. It's going to be about detecting the presence of devastating childhood disabilities."
"We are pleased to work with Dr. Beaudet and his team at Baylor on this critical project. This is a milestone achievement in prenatal research and for RareCyte, and we are looking forward to continued collaborations," said Ron Seubert, chairman and CEO of RareCyte.
"This work has moved a new prenatal genetic test closer to the clinic," said Beaudet. "We estimate that the test might be available to the public in one to two years."
More information: Amy M. Breman et al. Evidence for feasibility of fetal trophoblastic cell-based noninvasive prenatal testing, Prenatal Diagnosis (2016). DOI: 10.1002/pd.4924