Bacterial molecule trains the immune system to tolerate infection without inducing illness
Pathogen infection has been considered to have one of two general outcomes - either the infected organism develops some level of illness or its immune system fights off and eliminates the invading pathogen. In recent years, however, another outcome has been recognized; in some situations the infected organism, also called the host, can become tolerant to a specific pathogen, allowing the continued presence of a bacteria or virus without experiencing any negative effects. Originally identified and studied in plants, disease tolerance has more recently been recognized in animals, including humans.
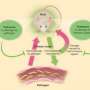
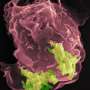
Now a study from Massachusetts General Hospital (MGH) investigators has identified a mechanism leading to tolerance of a common, difficult-to-treat bacteria by means of small molecule usually used by the pathogen to monitor its presence in the environment. In their report, which has received advance online publication in Nature Microbiology, the MGH team describes finding how excretion by Pseudomonas aeruginosa of this molecule called 2-aminoacetophenone (2-AA) that can essentially train the immune system of mice to allow the continued presence of the bacteria by reprogramming innate immune cells that normally would set off a pathogen-clearing inflammatory response. P. aeruginosa is a common cause of opportunistic infections, many strains of which have become resistant to most antibiotics.
"Our findings are unprecedented in that we have identified a specific bacteria-excreted molecule that acts as a host-tolerance/resilience training agent," says Laurence Rahme, MS, PhD, director of the Molecular Surgical Laboratory in the MGH Department of Surgery and senior author of the report. "These new insights may prove of enormous potential for developing preventive strategies to train host immune systems to become safely tolerant to pathogens and could provide avenues for the development of novel therapeutic interventions against bacterial infection."
For their investigation, Rahme's team focused on 2-AA a "quorum-sensing" molecule used by several bacterial species to monitor their density in the host environment. In previous work, her team had found evidence work indicating that 2-AA induce tolerance; for example, 90 percent of mice treated with 2-AA survived P. aeruginosa infection, compared with 10 percent of animals not treated.
Other research has suggested that pathogens can avoid host immunity through epigenetic reprogramming, which alters whether or not specific genes are expressed. One common epigenetic mechanism is the addition or removal of acetyl molecules from the histone proteins around which DNA is wrapped, and bacteria are known to manipulate host immunity by affecting enzymes that control the process of acetylation.
In a series of experiments Rahme's team first confirmed that pretreatment of human and mouse innate immune cells with 2-AA had long-lasting, suppressive effects on the inflammatory response. They also found that reducing acetylation in cells by 2-AA treatment suppressed the expression of inflammatory cytokines in both cellular and animal models. The expression and activation of HDAC1 (histone deacetylase 1), an enzyme that removes acetyl groups, was found to be elevated in 2-AA-treated cells, and inhibiting the activity of HDAC1 reduced tolerance to P. aeruginosa and increased mortality in mice.
Overall the results suggest that 2-AA essentially trains innate immune cells to accept the presence of certain pathogens by means of HDAC1, which prevents the transcription of inflammatory proteins. In addition, Rahme explains, secretion of 2-AA reduces the production by P. aeruginosa of the virulence factors responsible of the damaging effects of infection.
"Simply put, 2-AA modulates both the bacterium and the host," she says. "In the bacterium, it silences acute virulence functions and prompts bacterial cells to switch to a less metabolically active state. And by also dampening the host innate immune cells, 2-AA allows the bacteria to persist by preventing the inflammatory responses that also can damage host cells."
More work needs to be done to understand the molecular mechanisms allowing this mutually beneficial adaptation of both host and pathogen, explains Rahme, who is an associate professor of Surgery, Microbiology and Immunology at Harvard Medical School. "Our findings have the potential of revolutionizing our view of host defense and immunologic memory and may lead to new classes of vaccines and immunotherapies that could have a broad impact on human health worldwide."
More information: Arunava Bandyopadhaya et al, A quorum-sensing signal promotes host tolerance training through HDAC1-mediated epigenetic reprogramming, Nature Microbiology (2016). DOI: 10.1038/NMICROBIOL.2016.174