A pile of cocaine hydrochloride. Credit: DEA Drug Enforcement Agency, public domain
Researchers at the Medical University of South Carolina (MUSC) have used viruses to infect neurons with genes that allow them to switch on brain receptors involved in suppressing addiction relapse. Results of these preclinical studies were published in the September 28th, 2016 issue of the Journal of Neuroscience. The technology, called designer receptors exclusively activated by designer drugs, or DREADDs, is one of the most promising gene therapies for the future treatment of addiction in humans.
The brains of people who use cocaine become hijacked by drug cues. Powerful memories are formed between these cues-such as the using environment and drug paraphernalia-and the dopamine flood that occurs from using the drug itself. In users trying to quit, these drug cues activate an intense desire to seek cocaine again.
Resistance to relapse is partly mediated in the ventromedial prefrontal cortex-the brain region slightly above and behind our eyes, where previously learned associations are broken. This region of the brain stores extinction memory, which works to suppress the emotional response to drug cues, according to Jamie Peters, Ph.D., Research Assistant Professor in the MUSC Department of Neuroscience.
"Extinction doesn't overwrite the original memory," explained Peters. "It just helps suppress the pathological component of the response."
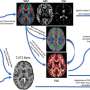
Peters and her colleague Peter W. Kalivas, Ph.D., Chair of the MUSC Department of Neuroscience, wanted to know if the response to drug cues associated with the dopamine rush of cocaine could be suppressed when the extinction memory region was activated. To test their hypothesis, they obtained viruses carrying the DREADD gene from Bryan L. Roth, M.D., Ph.D., in the Department of Pharmacology at the University of North Carolina Chapel Hill. The DREADD technology is openly accessible to researchers around the world through the National Institutes of Mental Health Psychoactive Drug Screening Program, where Roth serves as director.
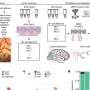
The viruses work by inserting the DREADD gene directly into the genome of cells, causing them to grow receptors on their surface that are normal except for a slight alteration. These receptors express a protein encoded by the DREADD gene that allows them to be activated by a single drug designed to bind that protein. In this case, the Peters lab infused a virus carrying a DREADD gene designed to change surface receptors on neurons. After the neurons were infected, they would fire in response to administration of the designer drug. Because the body's other cells had not been infected with the DREADD gene, they would remain unaffected.
"This new approach for treating drug addiction is exactly what is needed because it is targeted to a specific circuit in the brain regulating addiction," said Kalivas. "This may allow the circuit to be selectively regulated with minimum side effects on other circuits and brain functions."
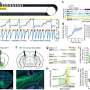
The researchers allowed rats to self-administer cocaine by pressing one of two levers, one active and one inactive. Once a rat pressed the active lever, cocaine was delivered along with a brief audio tone and a pulse of light that would serve as the drug cues. After a series of daily cocaine exposure sessions, the rats had learned to associate the simple drug cues with cocaine availability. Then they were removed from the drug. Next a surgical technician infused virus carrying the DREADD gene directly into the rats' ventromedial prefrontal cortices. After two weeks of cocaine abstinence, the rats were placed back in front of the two levers in ten daily sessions, but this time the levers produced neither cues nor cocaine. The next day, rats were subjected to a relapse test where the cues were returned. Before testing, half of the rats were given designer drug and half were not. Next, rats underwent an additional relapse test where they were given a low dose of cocaine to trigger relapse.
The experiments worked. Rats that were given the designer drug relapsed less in the presence of drug reminder cues. However, when exposed to cocaine again, rats relapsed regardless of whether they were given the designer drug. In other words, Peters' hypothesis was correct: rats with activated extinction memories weren't as susceptible to relapse triggered by cocaine-associated cues but were still vulnerable when exposed to cocaine again. This meant that extinction memory retrieval reduced relapse triggered by reminder cues.
This study shows that it is possible to use this technology to target a small population of cells in the brain that is important for regulating addiction, thereby inhibiting the drive to relapse to addictive drug use. In the future, Peters hopes that safe and effective viruses of this kind can be infused into the brains of human addicts during neurosurgery. A person would simply take a pill to activate the extinction memory region of their brain, helping them to suppress the urge to seek out drug in the face of those reminder cues. Since extinction memory isn't as powerful as the emotional response to a drug, this strategy could work when paired with effective psychological counseling approaches such as cognitive behavioral therapy.
Clinicians interested in using DREADDs in humans will have to remain patient, however. DREADDs have to be designed to match drugs that suppress only memories of drug cues while leaving other memories unaffected. And the crystal structure of newer human-appropriate designer drugs bound with the special receptors is being actively investigated in order to visualize exactly how they might work some day in patients with cocaine addiction.
"Certainly within my lifetime I would expect to see these virus-mediated gene therapies start to be used in the brain, in a neurosurgical setting," said Peters. "You can envision a person ultimately taking a pill to activate this very specific part of his or her brain."
More information: I. F. Augur et al, Chemogenetic Activation of an Extinction Neural Circuit Reduces Cue-Induced Reinstatement of Cocaine Seeking, Journal of Neuroscience (2016). DOI: 10.1523/JNEUROSCI.0773-16.2016
Journal information: Journal of Neuroscience
Provided by Medical University of South Carolina