Kathleen O'Connor, director of Markey's Breast Translational Group. Credit: University of Kentucky
University of Kentucky Markey Cancer Center Oncologist Dr. Edward Romond spent his career at UK treating and studying breast cancer, even leading major Phase 3 clinical trials on the breast cancer drug trastuzumab in the early 2000s. Commonly known as Herceptin, this drug became a standard of care for patients with HER2-positive breast cancer.
Though he retired from practice last year, Romond continues to work part-time with the research team at Markey, this time pushing toward a cure for a different, more deadly, type of breast cancer.
"Breast cancer, we now recognize, is at least five different disease that are completely different from each other," Romond said. "And the hardest nut to crack is this one called triple-negative breast cancer."
Triple-negative breast cancer is a moniker given to a particularly aggressive group of breast cancers that often affect younger women. Unlike the receptor-positive types of breast cancer, which have biomarkers that tell oncologists which treatment the patient should respond to, triple negative breast cancers have no definitive biomarkers. If the patient does not respond well to the current standard of care, it's up to the oncologist to make an educated guess about which chemotherapy will do the job.
The good news is that triple-negative breast cancers do generally respond well to chemotherapy. However, because triple-negative breast cancers are not homogenous, and every single patient responds differently to various chemotherapies, it's difficult to predict which chemotherapy will best treat each patient's cancer.
But the researchers at the UK Markey Cancer Center are working to change that paradox. Markey's Breast Translational Group is currently developing a proposed clinical trial that could create a major shift in the way triple-negative breast cancers are treated.
Currently, after a patient is diagnosed with triple-negative breast cancer, she usually receives chemotherapy first to try and shrink the tumor (known as neo-adjuvant therapy), followed by surgery to remove as much of the mass as possible. The patients are then monitored for signs of recurrence. If a patient has residual cancer despite getting neoadjuvant chemotherapy, they are at a high risk for recurrence.
There are currently at least six different types of chemotherapy that can be used as a possible therapy for patients, and each one may affect each individual patient in a different way. To tailor the treatment to each distinct patient, the investigators aim to test the tumors in a set of animal model "avatars" with these different therapies to gauge the response.
Here's how the proposed trial would work: after the patient's biopsy, her cancerous tissue would be transferred into a mouse that is bred to grow human tumors, then subsequently into three dozen mice: her "avatars." While the patient undergoes neo-adjuvant chemotherapy and then surgery – a process that can take up to six months – the avatars will be divided into groups, with each group receiving one of the six available chemotherapies.
Triple-negative breast cancer can be difficult to treat, but a new clinical trial currently in development at the University of Kentucky Markey Cancer Center could potentially change the standard of care for this deadly disease. Credit: University of Kentucky
When the researchers see which avatar group has the best result, they'll know which chemotherapy should work best for that patient. Knowing this would provide additional options for women who have residual cancer after neoadjuvant chemotherapy, and may reduce their risk for disease recurrence.
"It would prevent us from having to experiment with each individual patient, and end up finding that they didn't respond to that therapy," said Kathleen O'Connor, director of Markey's Breast Translational Group. "If we can do this, then the oncologists will no longer have to guess."
Dr. Aju Mathew, a medical oncologist who treats triple-negative breast cancer patients at Markey, compares his team's game-changing proposition to the way Uber has altered the use of public and personal transportation.
"We often hear about disruptive technology—Uber being one, for example," he said. "It disrupted the current paradigm of everyone driving a car on their own or hiring a cab. This trial is our way of disrupting the current standard of care, the current technology, and the current practice of medicine, to try to change the paradigm of 'one size fits all' approach for triple-negative breast cancer patients."
Though the avatar model of research isn't new, O'Connor notes that not many researchers are using them specifically for the treatment of an individual patient. Using a trial protocol to get the tissues directly from the patient's biopsy is a key factor in making the research work.
"The important thing is that we need to get the tumor tissue before they've been exposed to chemotherapy," O'Connor said. "This is one of the things that makes our trial unique."
With the trial design in place, the team just needs to provide ample data showing that growing a patient's tumor in the avatar from biopsy will work. But to gather that data, they need more funding. Initial pilot funds stemming from Markey's National Cancer Institute (NCI) designation grant have enabled the team to establish their first set of avatars with tissues taken from patients' surgeries. But a boost in funding would help them establish the preliminary data for the trial and allow the team to then apply for major federal funding.
"We have a large group of people who have freely given their time up to this point," O'Connor said. "But we need to have money to protect the time of the researchers doing this work, and we need enough money to get the mice in order to do this."
Funding for triple-negative breast cancer has been a major focus for Lexington resident Cindy Praska, whose daughter Whitney was diagnosed with the disease in 2007 at age 24. After undergoing a double mastectomy, chemotherapy and radiation at another hospital, Whitney was deemed cancer-free.
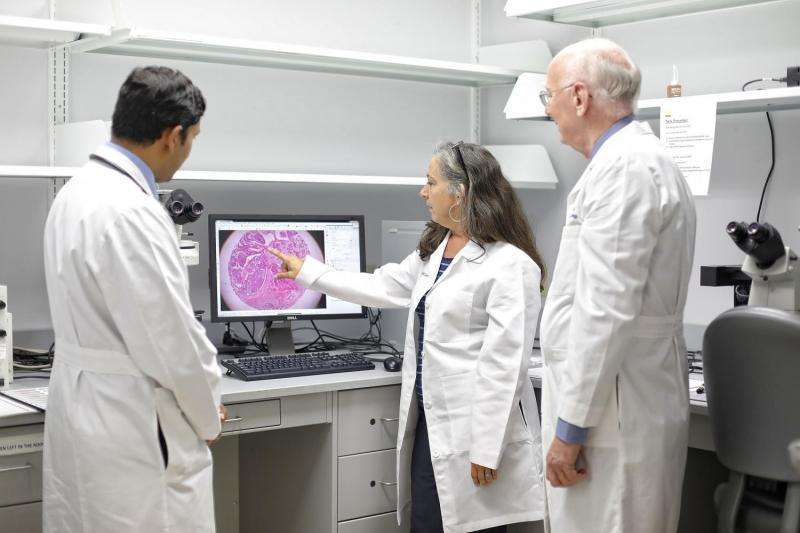
Dr. Aju Mathew, Kathleen O'Connor and Dr. Edward Romond view pathology images in the lab. Credit: University of Kentucky
In the years following her diagnosis, Whitney became an advocate for breast cancer awareness and fundraising, becoming actively involved in the Frankfort Country Club's Rally for the Cure, which has raised money for the Susan G. Komen Foundation and the University of Kentucky Markey Cancer Center for nearly 20 years.
Though her initial treatment for triple-negative breast cancer was successful, Whitney then developed osteosarcoma in 2012. This time, she elected to have her surgery out of state and came to Markey for her chemotherapy. Genetic testing revealed she carried a P53 genetic mutation, which was the cause of her original cancer, and combined with the radiation she had received prior, also caused her osteosarcoma. Despite Whitney's and her doctors' best efforts, her cancer metastasized and she succumbed to the disease in November of that year.
Carrying the torch for her daughter, Cindy continues to push for education, awareness, and research toward triple-negative breast cancer and is still heavily involved in fundraising.
This Saturday, Cindy and the team behind the Frankfort Country Club Rally for the Cure have planned a "party with a purpose" called Bourbon & Jazz for the Cure to celebrate the organization's 20th anniversary. Held at the Frankfort Country Club on Saturday, Oct. 15 at 6:30 p.m., this special fundraising gala includes a silent and live auction featuring limited-edition Buffalo Trace bourbon bottles, and the funds raised from the gala will directly benefit the research team behind this proposed clinical trial.
"Whitney helped bring awareness to this disease, and it is so rewarding to me that work is progressing so that more young women her age will live to marry, have a family, and be able to see their young children grow up," Cindy said. "It has given me a purpose to be an advocate for these causes and it's an honor to be supporting Markey, who we called family and home the last year of her life."
Provided by University of Kentucky