Discovery of stem cell signals could help regenerate bones missing in congenital skull disorders

Calcium, the main constituent of bone, turns out to play a major role in regulating the cells that orchestrate bone growth, a finding that could affect treatment for conditions caused by too much collagen deposition, such as fibrosis and excessive scarring, as well as diseases of too little bone growth, such as Treacher Collins Syndrome (TCS).
The finding by Michael Rape and his colleagues at the University of California, Berkeley, came from study of the signals that tell undifferentiated stem cells in the very early embryo to mature into bone cells. In the craniofacial disorder TCS, for example, the embryo does not form a structure called the neural crest, from which the jaws, inner ear and numerous other bones in the head and face develop.
As a result, people like Francis Smith, a 41-year-old University of Colorado researcher who visited Rape in his lab a month ago, require dozens of surgeries during childhood to reconstruct the face, implant hearing aids and even reconstruct the trachea to breathe normally.
Rape hopes that basic research to pinpoint the key signals that trigger proper bone growth can help those like Smith avoid such painful surgeries. One option could be the implantation of a biodegradable matrix seeded with bone cells called chondrocytes, which would then be stimulated to release collagen, the blueprint for bone growth. The new findings suggest that stimulating collagen release with calcium would also trigger proper bone growth.
"You would basically add calcium to cells on those support structures, which is fairly easy, and motivate chondrocytes to secrete the collagen that is needed to build a bone structure on top of that support," said Rape, the Dr. K. Peter Hirth Chair in Cancer Biology in the Department of Molecular and Cell Biology and an investigator at the Howard Hughes Medical Institute. "That would be exciting, but it is very much in the future. Nevertheless, this might become a possibility the more we understand about how cells make their decisions."
The finding could also explain how messing with the body's calcium levels during pregnancy can cause facial deformities such as those associated with fetal alcohol syndrome.
Rape and his colleagues reported their results online Oct. 6 in the journal Cell.
How cells make decisions
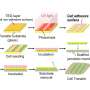
Using a unique screening process he developed, Rape searches for enzymes that attach a protein called ubiquitin to other proteins inside stem cells, driving these undifferentiated cells toward a specific fate. Attaching one molecule of ubiquitin to a protein often suffices to trigger a cell-fate decision, such as forcing a pluripotent stem cell to become a collagen-producing chondrocyte. Enzymes that attach a chain of ubiquitin molecules to a protein often target the protein for destruction and recycling - the type of autophagy that was recognized by this year's Nobel Prize in Physiology or Medicine.
Last year, Rape's lab identified an enzyme, called CUL3, that adds ubiquitin to the protein TCOF1 and makes stem cells mature into neural crest cells. When both copies of TCOF1 are mutated, the embryo dies. But if only one of the two copies of TCOF1 is mutated, as in TCS, fewer neural crest cells are produced and many die early, leading to improper bone growth in the skull.
Other studies have shown that CUL3 acts at many stages of human development, and problems with regulation of CUL3 have been associated with autism, schizophrenia, myopathy and hypertension.
In his new experiments, Rape searched for the signals that turn on ubiquitylation enzymes like CUL3, allowing the developing embryo to form bone at the right time and in the right place in the organism. He found that CUL3 needs the help of two calcium-binding proteins, which was a surprise.
"Our research basically identifies calcium not only as a structural element of bone, which makes the bone strong and sturdy, but also as a signaling molecule for bone formation that we hadn't appreciated before, which can be used to turn enzymes on and off," he said. "Calcium is a very important regulatory molecule that allows the organism to make cell-fate decisions."
Because CUL3 not only triggers stem cells to become neural crest cells, but stimulates chondrocytes to secrete collagen, calcium evidently plays a major role in many aspects of bone formation.
"This means is that you basically have many different steps that come together in order to form a bone, and that they are beautifully orchestrated by calcium," Rape said. "Calcium is really a sort of integrating signal, which is there at the right time and place in development to allow the developing organism to set in motion a whole series of reactions to build the bone where it should be built. It is a very beautiful idea that you don't need a lot of different signals to come together, but you use one that really pops into place the right sequence of events."
Further research is needed, however, before physicians can attempt regenerative therapies to help those like Smith who are born with TCS.
"The more you understand about each of these steps, the easier it is to focus your applied research onto things that matter and change something for these patients," Rape said.